As Philips, we are committed to helping caregivers treat their patients better. Playing a central role in the transition from open to minimally-invasive interventions was already a great step forward in this. Now we are on a research journey to further innovate image guided procedures.
Click on the icons to see the presentations/papers/videos
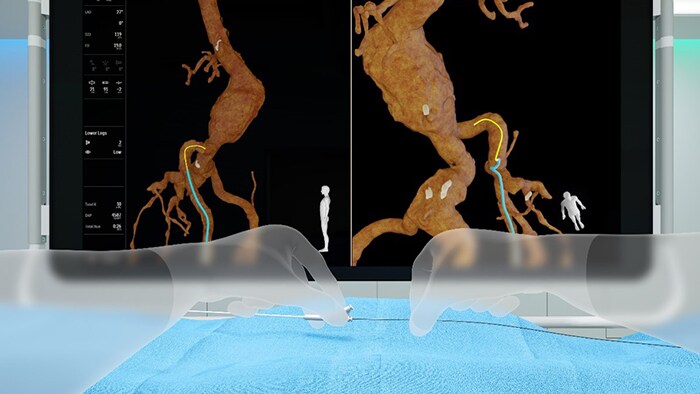
We want to break through the current limitations of image guided therapy so clinicians can reduce their dependency on fluoroscopy while seeing intra-body devices ever more clearly. In line with this ambition, our Fiber Optic RealShape (FORS) technology sparks a new era in device guidance. This unique technology enables real-time 3D visualization of the full shape of devices inside the body without the need for fluoroscopy.
Key FORS moments as shared by the Limited Edition sites during CX Aortic 2021
Why push beyond the limitations of minimally invasive therapy?
As the transition from open surgery to minimally-invasive interventions continues, more and more procedures are being carried out using this approach. One consequence is that increasingly complex interventions can take place in a minimally-invasive way. However, this also has a downside; longer procedure times, intense training programs, higher radiation exposure and an increased usage of contrast agent. All of which brings potential health risks to patients, physicians and staff. X-ray is considered the ‘gold standard’ for the imaging used to guide physicians during such interventions, because of its ability to visualize devices, in real-time, within the anatomy. Yet navigation within complex anatomies remains a challenge, even when the 2D X-ray device visualization is combined with 3D anatomical information as an overlay. As clinicians continue to expand their practice and strive for better and more effective ways to treat patients, there is a growing need for them to see devices in 3D, in real time, and in relation to the anatomy. At the same time, they want to reduce exposure to radiation for themselves, their staff and their patients. At Philips, we have already made progress in tackling this visualization and radiation reduction need. Building on this experience – and after extensive dialogue with medical professionals on the frontline of this specialized area of healthcare – we concluded that something completely new was required. Not incremental innovation, but rather a breakthrough technology. One that opens up new possibilities in carrying out minimally-invasive procedures, standard or complex, through intuitive device and anatomy visualization in a radiation-free environment.
What is FORS?
Device guidance using light
Fiber Optic RealShape (FORS) is a ground-breaking technology platform which enables real-time 3D visualization of the full shape of devices inside the body, without the need for stepping on the fluoro pedal. This technology platform consists of equipment which sends pulses of light through hair-thin optical fibers that run within minimally invasive devices. This platform exclusively integrates with our Philips interventional X-ray systems. The FORS technology has the following characteristics: Following successful pre-clinical trials, the FORS FIRST Clinical Study was executed at the University Medical Center of Utrecht, The Netherlands. This was a major milestone on our research journey. These results were shared during the LINC 2019 congress in Leipzig, Germany and further communicated at key congresses in 2019 and 2020. Please refer to the end of this page to link to all these presentations. Following CE mark for our Limited Edition, and the further clearance from the FDA, we have started the next phase of our research journey. With selected partner hospitals from Europe and the USA, we are conducting clinical studies with the initial focus to learn and perfect our technology within vascular procedures – specifically endovascular aortic repairs. Representative procedures are shared by the Limited Edition sites, at major clinical congresses since 2020.
How does FORS work?
Through FORS technology, devices are visualized inside the body using the ingenious application of light travelling through hair-thin optical fibers. The technology is based on the concept of measuring strain in optical fibers, using light reflected from density fluctuations in these fibers.
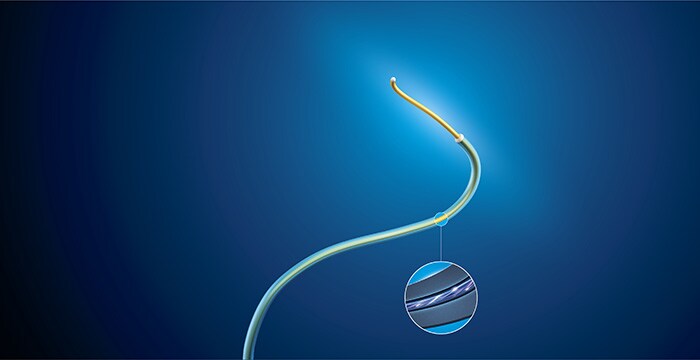
To achieve this, an optical fiber is integrated into intra-body devices. By sending laser light into this fiber and then analyzing how it is reflected back along the fiber, we can reconstruct and visualize the full shape of the devices. In 3D. In real time. In distinctive colors. And from any viewing angle. These intra-body devices can be shown in the context of the patient’s anatomy through integration with images obtained by conventional pre- or intra operative techniques (CT, MRI and X-ray fluoroscopy respectively). Clinicians can therefore see with clarity where and how they need to navigate and position their devices within the anatomy. FORS is a prime example of how we have combined our optics and device legacy with our image guidance expertise to create a technology that is at the intersection of imaging guidance and therapy.
For complimentary information, please do not hesitate to reach-out to: FORS_inquiries@philips.com
FORS Limited Edition is CE marked and 510(k) cleared.
Building the continuum of evidence
Technical studies
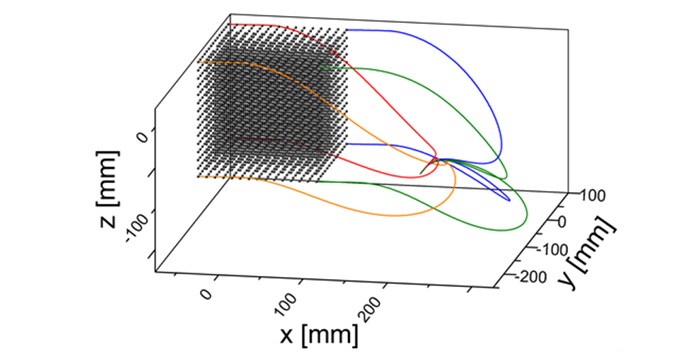
Bench accuracy measurement
FORS visualization
Pre-clinical studies
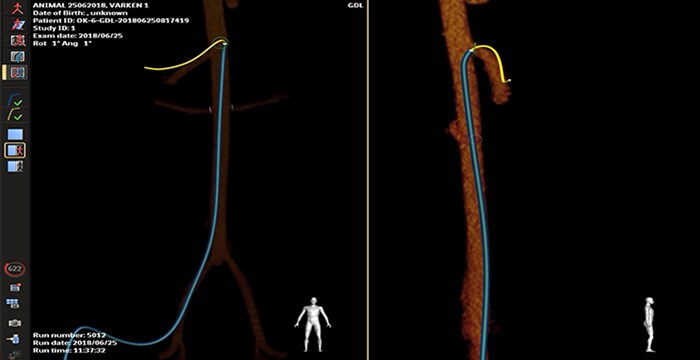
Porcine SMA cannulation
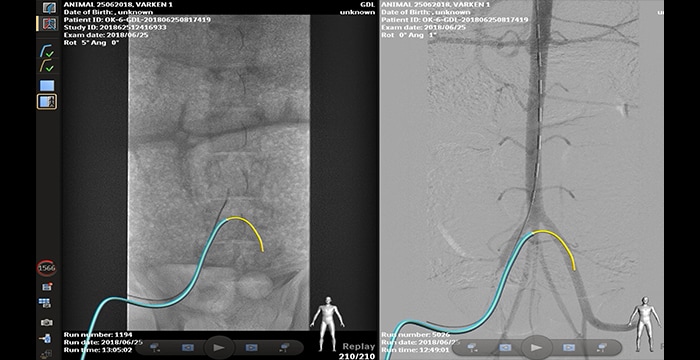
Porcine aorto-iliac cross-over
First-in-Human, single center, feasibility study
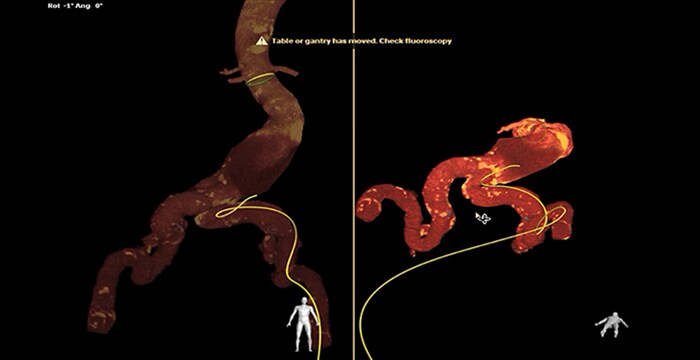
Navigation through tortuous iliac artery
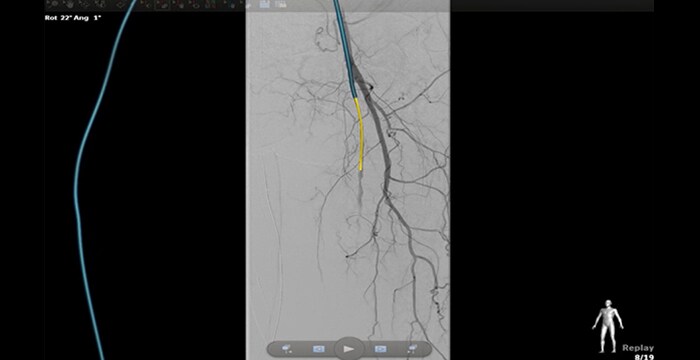
Navigation through stenotic vessels
Multi-center studies from selected partner hospitals (EU/USA)
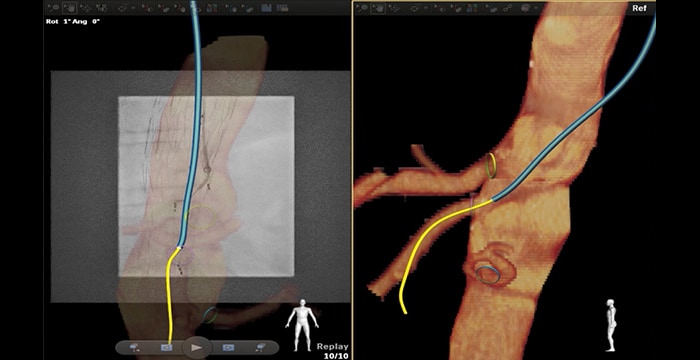
Catheterization SMA through fenestrated EVAR with brachial access
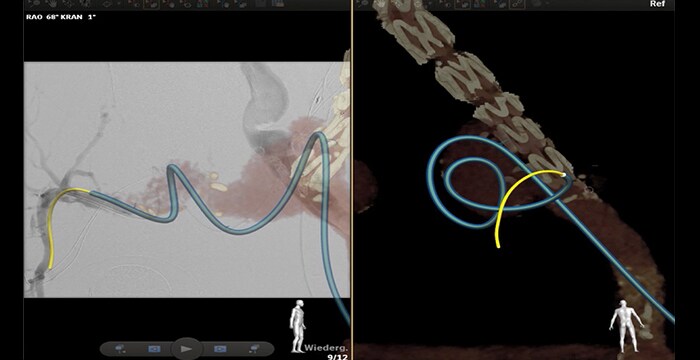
Catheterization of the right internal iliac artery crossing an aneurism
For all case studies shared here: Results from case studies are not predictive of results in other cases. Results in other cases may vary.