Cardio-oncology: Ultrasound’s role in detecting cardiotoxicity
A 2019 report by the American Cancer Society shows that there are approximately 17 million cancer survivors in the United States.1, 2 Although new, potent, and targeted cancer therapies are to thank for this increasing survival rate, they are also behind one of the leading mortality causes amongst cancer patients: cancer therapy-induced cardiovascular disease.3 The long-term consequences of cancer treatment are at the genesis of the cardio-oncology field: an evolving multidisciplinary sub-specialty. It is focused on detecting, monitoring and treating cardiovascular dysfunction that occurs as a side effect of chemotherapy, radiotherapy, or other potentially cardiotoxic cancer-directed therapies while taking into account individual risk factors for developing certain cardiovascular (CV) complications. Despite the lack of a clear evidence-based approach on how to detect cardiotoxicity, define or classify it, there are key factors that seem to gather consensus: time and accuracy in the identification of cardiotoxicity are crucial. This article focuses on the importance of early and accurate detection of cardiac dysfunction and how advanced imaging modalities can help clinicians with exactly that.
Cardiotoxicity: a definition
Cardiotoxicity can be defined as any functional or structural heart injury related to cancer treatment. Chemotherapy, radiotherapy and targeted therapy may all cause cardiotoxicity and cancer therapy-related cardiac dysfunction (CTRCD).
"Beyond oncologic therapies, cancer and CV diseases are connected by complex pathophysiological mechanisms and shared modifiable and non-modifiable risk factors that may increase the potential for cardiotoxicity.4,5 For that reason, a comprehensive cardiovascular evaluation and monitoring, throughout the cancer process, is needed32"
- Dr. Teresa López-Fernández
La Paz University Hospital, CIBER CV. IdiPAZ Research Institute
Cardiotoxicity: Signs, detection and surveillance strategies
In the cardiotoxicity detection arena, a lot has changed following the 1960’s recognition of the side effects of anthracyclines in cardiac dysfunction.6 Several strategies to detect cardiotoxicity have been used — X-ray, endometrial biopsy, blood tests, electrocardiogram, echocardiogram7 – each with its merits and limitations. Signs and symptoms of cardiotoxicity may include chest pain, changes in heart rhythm, shortness of breath, and ankle swelling8. However, heart function can also decline without any noticeable signs of cardiotoxicity and some of these may not be present until months or years after the cancer treatment. The challenges posed by a nonexistent universal definition of cardiotoxicity and CTRCD identification guidelines are evident9. The common indicator that brings organizations and experts together? A serial decline in left ventricular ejection fraction (LVEF), defined using a threshold change in LVEF. One of the most recent comes from the American Society of Echocardiography’s Expert Consensus report. It defined cardiotoxicity as an LVEF drop ≥10% from baseline to a value of <53% which is confirmed by both repeated cardiac imaging, performed 2-3 weeks after the initial decrease,6 and GLS >15%
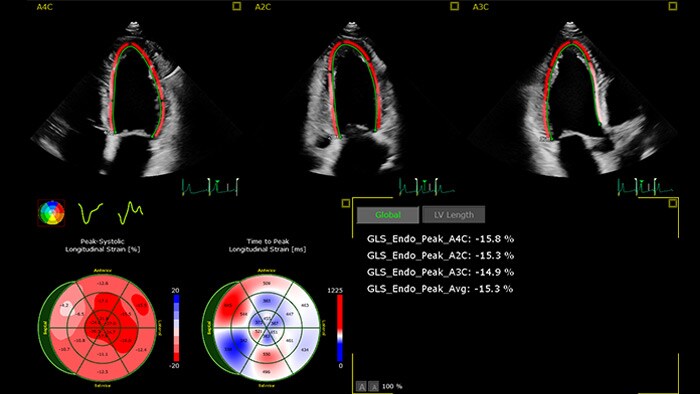
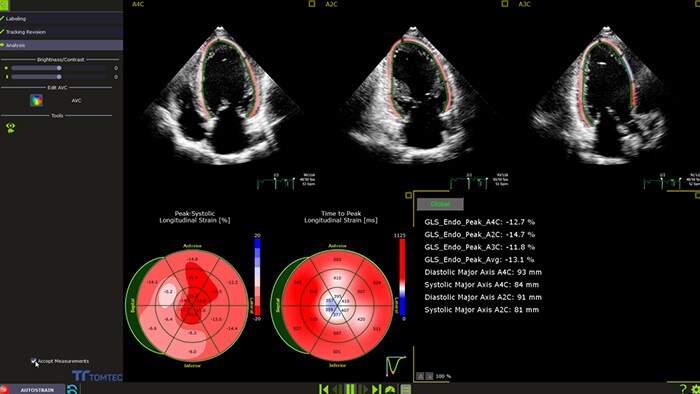
How to detect cardiotoxicity: the ultrasound’s role?
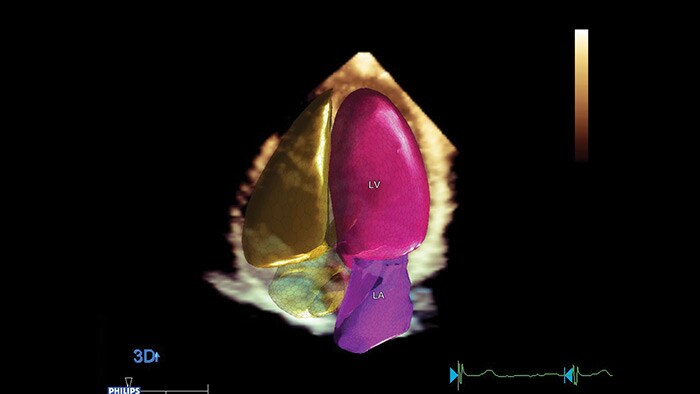
Non-invasive and accurate surveillance of LVEF by cardiac imaging ‘has emerged as the most widely used strategy for monitoring the changes in cardiac function, both during and after the administration of potentially cardiotoxic cancer treatment'.6 Recent publications 10 recognize advanced cardiac imaging for its growing accuracy and reproducibility as well as the personalization it offers through emerging imaging biomarkers and their potential to provide valuable insights in the interdisciplinary field of cardio-oncology. Among those emerging modalities are 2D and 3D echocardiography, global longitudinal strain (GLS) and cardiac magnetic resonance (CMR), cardiac biomarkers, and multi-gated acquisition scan (MUGA).
We spoke with Dr. Teresa López-Fernández about how cardiac ultrasound can help clinicians in detection, management, and follow-up cardio-oncology patients.
Five questions on the role of cardiac imaging in early detection of cardiotoxicity due to anticancer therapies
While the merits of echocardiography in detecting CTRCD in patients with cancer are recognized, there remain real challenges for it in the cardio-oncologic setting – such as image quality, accuracy, costs, availability and the need to train specialists to help in clinical decision-making. Philips cardiovascular ultrasound applications aim to bridge that gap. Powered by anatomical intelligence and automation technologies, Philips combines 3D imaging with fast and reproducible GLS measurements, for better accuracy, in less time. Accurate baseline echocardiography exams, follow-ups, and change assessments are easy to visualize and edit by experts and new operators – all in the same machine, no extra equipment required. Our commitment: delivering high-quality critical cardiac measurements in seconds. Better therapy management and planning for more patients in less time to help clinicians quickly and confidently assess cardiotoxicity symptoms and guide their selection of appropriate therapies.
Reviewed by: Dr. Teresa Lopéz-Fernández. La Paz University Hospital, CIBER CV. IdiPAZ Research Institute
Sources
1 https://www.cancer.org/latest-news/population-of-us-cancer-survivors-grows-to-nearly-17-million.html 2 https://acsjournals.onlinelibrary.wiley.com/doi/full/10.3322/caac.21565 3 https://academic.oup.com/eurheartj/article/40/48/3889/5637730 4 Ameri P, Canepa M, Anker MS et al. Heart Failure Association Cardio-Oncology Study Group of the European Society of Cardiology. Cancer diagnosis in patients with heart failure: epidemiology, clinical implications and gaps in knowledge. Eur J Heart Fail 2018;20:879-887 5 de Boer RA, Meijers WC, van der Meer P et al. Cancer and heart disease: associations and relations. Eur J Heart Fail 2019 Jul 18. doi: 10.1002/ejhf.1539 6 https://www.onlinejase.com/article/S0894-7317%2814%2900534-3/pdf#page=2 7 https://www.nccn.org/patients/resources/life_with_cancer/managing_symptoms/cardiac_toxicity.aspx 8 https://ascopubs.org/doi/10.1200/JCO.2016.70.5400 10 https://doi.org/10.1093/eurheartj/ehw211 11 López-Fernández T, Martín García A, Santaballa Beltrán A et al. Cardio-Onco-Hematology in Clinical Practice. Position Paper and Recommendations. Rev Esp Cardiol 2017;70:474–486 12 Zamorano JL, Lancellotti P, Rodriguez Muñoz D et al. 2016 ESC Position Paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC Committee for Practice Guidelines. Eur Heart J 2016;37:2768–2801 13 López-Fernández T, Thavendiranathan P. Emerging cardiac imaging modalities for the early detection of cardiotoxicity due to anticancer therapies. Rev Esp Cardiol. 2017;70:487-495 14 Plana JC, Thavendiranathan P, Bucciarelli-Ducci C, Lancellotti P. Multi-Modality Imaging in the Assessment of Cardiovascular Toxicity in the Cancer Patient. JACC Cardiovasc Imaging. 2018;11:1173-86 15 Asteggiano R, Aboyans V, Lee G et al. Cardiology care delivered to cancer patients. The results of a questionnaire survey by the Council for Cardio Oncology and Council for Cardiology Practice of the European Society of Cardiology. CardioPulse. European Heart Journal 2020; 41 (2): 205–206.https://doi.org/10.1093/eurheartj/ehz935 16 Mitroi C, Martín-García A, Mazón Ramos P et al. Current functioning of cardio-oncology units in Spain. Clin Transl Oncol. 2019 Dec 20. doi: 10.1007/s12094-019-02250-4. [Epub ahead of print] 17 Lancellotti P, Galderisi M, Donal E et al. Protocol update and preliminary results of EACVI/HFA Cardiac Oncology Toxicity (COT) Registry of the European Society of Cardiology ESC Heart Fail. 2017 Aug;4(3):312-318 18 Rebecca L. Siegel, Kimberly D. Miller, Ahmedin Jemal, DVM, Cancer Statistics, 2020. CA Cancer J Clin 2020;70:7-30 19 Jessup M, Bozkurt B, Butler J et al. 2017 ACC/AHA/HFSA Focused Update of the 2013 ACCF. Circulation 2017;136:e137-e161 20 Ali MT, Yucel E, Bouras S et al. Myocardial Strain Is Associated with Adverse Clinical Cardiac Events in Patients Treated with Anthracyclines J Am Soc Echocardiogr. 2016 Jun;29(6):522-527.e3. 21 Oikonomou EK, Kokkinidis DG, Kampaktsis PN et al. Assessment of Prognostic Value of Left Ventricular Global Longitudinal Strain for Early Prediction of Chemotherapy-Induced Cardiotoxicity: A Systematic Review and Meta-analysis. JAMA Cardiol. 2019 Aug 21. doi: 10.1001/jamacardio.2019.2952. 22 Kenigsberg B, Wellstein A, Barac A et al. Left Ventricular Dysfunction in Cancer Treatment: Is it Relevant? JACC Heart Fail. 2018 Feb;6(2):87-95. 23 Curigliano G, Cardinale D, Dent S, Criscitiello C, Aseyev O, Lenihan D, Cipolla CM. Cardiotoxicity of anticancer treatments: Epidemiology, detection, and management. CA Cancer J Clin. 2016 Jul;66(4):309-25 24 Cardinale D, Colombo A, Lamantia G, et al. Anthracycline-induced cardiomyopathy. Clinical relevance and response to pharmacologic therapy. J Am Coll Cardiol 2010;55:213–220. 25 Zhang KW, Finkelman BS, Gulati G et al. Abnormalities in 3-Dimensional Left Ventricular Mechanics With Anthracycline Chemotherapy Are Associated With Systolic and Diastolic Dysfunction.JACC Cardiovasc Imaging. 2018 Aug;11(8):1059-1068. 26 Triposkiadis F, Butler J, Abboud FM et al. The continuous heart failure spectrum: moving beyond an ejection fraction classification.Eur Heart J. 2019 Jul 1;40(26):2155-2163 27 Negishi T, Thavendiranathan P, Negishi K, Marwick TH et al. Rationale and Design of the Strain Surveillance of Chemotherapy for Improving Cardiovascular Outcomes: The SUCCOUR Trial. JACC Cardiovasc Imaging. 2018 Aug;11(8):1098-1105 28 Jessup M, Bozkurt B, Butler J et al. 2017 ACC/AHA/HFSA Focused Update of the 2013 ACCF. Circulation 2017;136:e137-e161 29 Edward H Romond, Jong-Hyeon Jeong, Priya Rastog et al. Seven-year Follow-Up Assessment of Cardiac Function in NSABP B-31, a Randomized Trial Comparing Doxorubicin and Cyclophosphamide Followed by Paclitaxel (ACP) With ACP Plus Trastuzumab as Adjuvant Therapy for Patients With Node-Positive, Human Epidermal Growth Factor Receptor 2-positive Breast Cancer. Clin Oncol 2012 Nov 1;30(31):3792-9. 30 Mohammed T Ali , Evin Yucel , Souhila Bouras et al. Myocardial Strain Is Associated With Adverse Clinical Cardiac Events in Patients Treated With Anthracyclines. J Am Soc Echocardiogr 2016 Jun;29(6):522-527.e3. 31 Lancellotti P, Suter TM, López-Fernández T et al. Cardio-Oncology Services: rationale, organization, and implementation: A report from the ESC Cardio-Oncology council. Eur Heart J. 2019 Jun 7;40(22):1756-1763 32 Mehta LS, Watson KE, Barac A et al. Cardiovascular Disease and Breast Cancer: Where These Entities Intersect. A Scientific Statement from the American Heart Association, American Heart Association Cardiovascular Disease in Women and Special Populations Committee of the Council on Clinical Cardiology; Council on Cardiovascular and Stroke Nursing; and Council on Quality of Care and Outcomes Research. Circulation. 2018;137:e30–e66