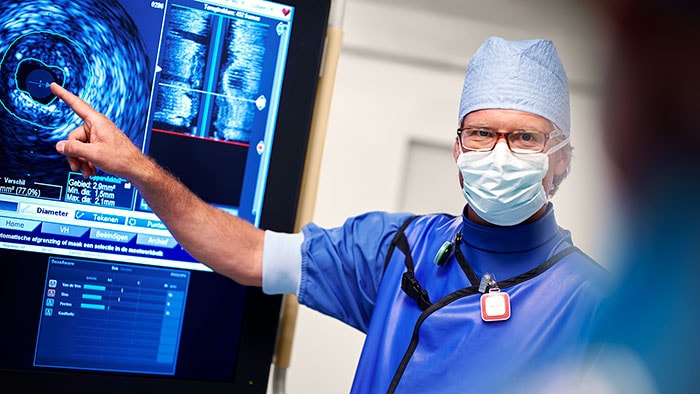
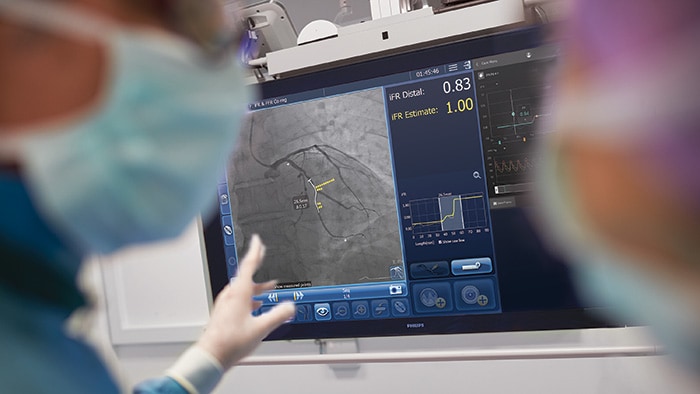
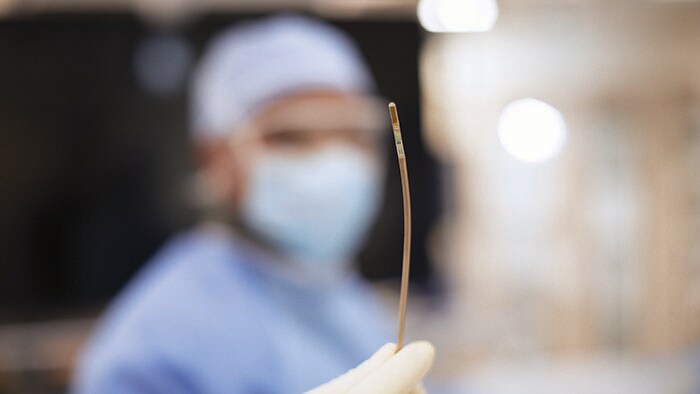
Atrial fibrillation (AFib) is, quite rightly, regarded as a global public health problem due to its devastating clinical and economic impact resulting from its association with embolic stroke, congestive heart failure and acute coronary syndrome. It’s thought around 33.5 million people worldwide suffer from AFib, a number probably underestimated due to difficulty diagnosing the often transient nature of a patient’s arrhythmia. It’s an issue that isn’t going anywhere: the number of those affected is likely to double by 2060, a jump that can be attributed to many factors, including increased incidence, greater survival after the onset of AFib and enhanced detection. Wearable devices will play a key role in increasing the rate of diagnosis but are also sparking debate over risks of overtreating, and whether it’s even necessary to treat all asymptomatic patients.
Wearable devices will play a key role in increasing the rate of diagnosis but are also sparking debate over risks of overtreating, and whether it’s even necessary to treat all asymptomatic patients.
The unstoppable rise of wearables
Wearables are now a staple of modern life, with the global market set to double by 2022. This lucrative (tipped to be worth well over USD 27 billion) market reflects the growing desire of consumers for more access to their own health data. Companies are investing heavily in exploring how next-generation wearables could help flag possible health problems, but also guide interventions to prevent disease. Last year, the FDA cleared a new ECG app as a class II medical device. It is intended for use with the Apple Watch to record the timing and strength of the electrical signals that make the heart beat. It was swiftly followed by AliveCor’s mobile electrocardiogram (ECG) device that promises a medical-grade ECG anytime, and can detect both AFib and other arrhythmias that could indicate heart disease. These wearables, and the others that have followed including those measuring blood pressure, sleep, and more, herald the growing trend for wearables that play a role in furthering awareness and detection of cardiovascular conditions.
Wearables in the spotlight
The Apple Heart Study, a virtual study with more than 400,000 participants, highlighted the potential role of wearables in detecting AFib. In the study, repeated detection of an irregular pulse within a 48-hour period triggered a notification to be sent via the app. Participants receiving a notification were prompted to contact the study doctor to determine the need to wear an ECG patch for up to one week. Initial study findings, shared at the American College of Cardiology (ACC) Scientific Sessions in March, supported the hypothesis that it could detect AFib in a small group of people who had received an alert of an irregular heartbeat. But while increased awareness and earlier diagnosis of AFib is to be welcomed, there is also concern that more information, with associated inevitable false-positives from wearable devices, could cause unnecessary anxiety and testing. So, while doctors are excited about the possibility of wearable health sensors, we also need the reassurance of the clinical data before we rely on it. In fact, data published in a 2017 study concluded that one-lead ECG screening tests have given a false diagnosis between 5% and 24% of the time. While false positives continue to improve with each generation of device, a sizable number of healthy patients will inevitably end up undergoing unnecessary testing. While physicians are well-accustomed to this dilemma with the myriad of screening tests we perform, still more needs to be done to understand the risks and benefits of AFib screening in asymptomatic individuals of all ages.
From wearables, to imaging systems, devices, and soon KODEX-EPD, physicians will have more tools than ever to help us decide, guide, treat and confirm AFib treatment. The future is bright for AFib patients.
Why is this important? The future of clinical trials
While there’s room for improvement in accuracy, the bigger story for me is that the Apple Heart Study was a proof of concept — a paradigm shift in how clinical trials can be conducted ‘virtually’ by removing the need for participants to physically visit a clinic. This sort of frictionless data collection on a massive scale is big news. It allows for larger data sets and fast clinical trial times. Indeed, the randomized controlled Heartline study targeting individuals over the age of 65, is set to launch later this year. Heartline will combine data from Johnson & Johnson’s heart health app with the Apple Watch 4’s ECG app to assess if wearables can successfully accelerate the diagnosis of asymptomatic AFib and, most importantly, improve outcomes by preventing stroke, myocardial infarction and death. With plans to enroll 180,000 patients, the Heartline study could be a pivotal study. Using wearables, participants can be found through media or their insurer, enlarging the recruitment funnel. Essentially, it’s a virtual trial, meaning that it can be done at a much larger scale, at a significantly lower cost.
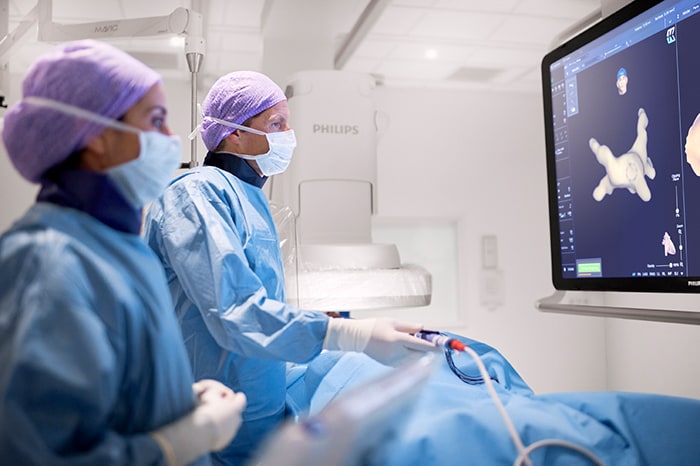
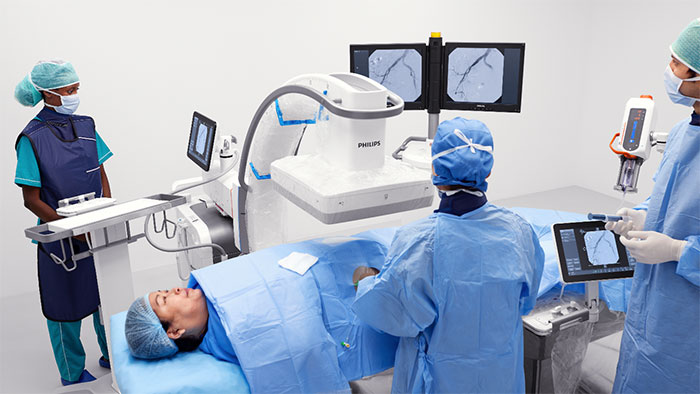
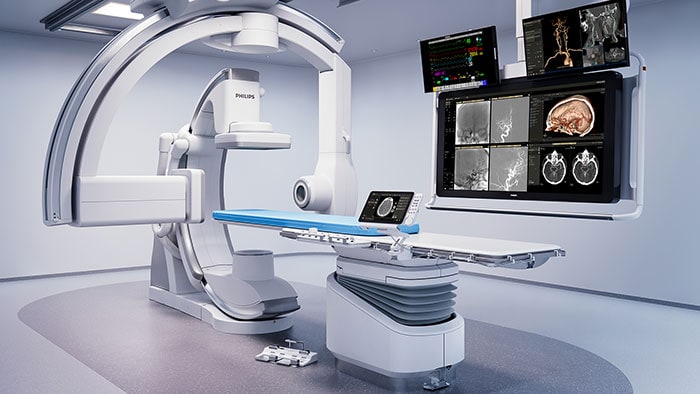
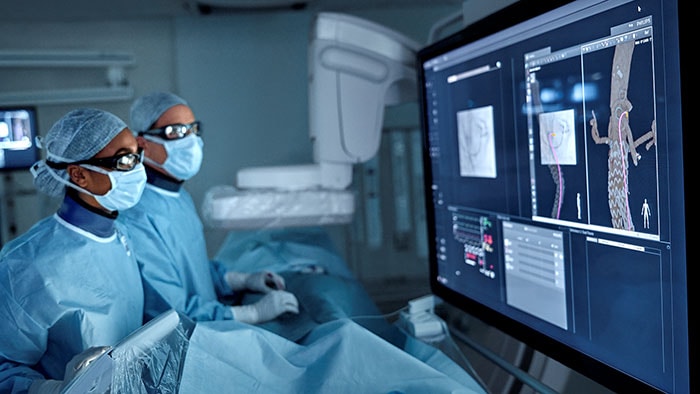
What’s next for AFib?
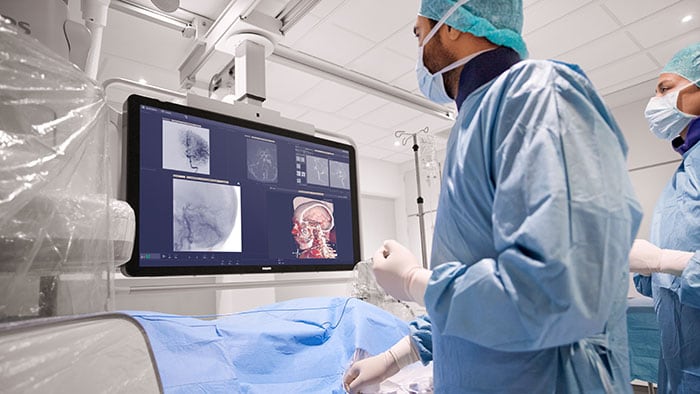
Current treatments for AFib have their limitations. Patients are often given anticoagulants or antiarrhythmic drugs, but these medications can be ineffective or not tolerated by patients. In many cases catheter ablation may be recommended as the next line of defense. Unfortunately, up to 40% of patients who have undergone ablation will suffer recurrence, necessitating a second treatment. Ablation is a nifty image-guided therapy procedure that builds upon the older, open-heart “Maze procedure” many of us remember. Today, electrophysiology (EP) physicians advance tiny ablation catheters to the left atrium via the femoral vein, traversing the intra-atrial septum using real-time x-ray and ultrasound guidance. They then use sophisticated 3D mapping systems to define targets around the pulmonary veins which are the source of erratic electrical signals causing AFib. They then use either cryoablation (cold) or radiofrequency ablation (heat) to scar the tissue around the circumference of the pulmonary veins (hence the term pulmonary vein isolation), interrupting these erratic electrical pathways. And, remarkably, the patient leaves the interventional suite with just a small bandage at the groin. We have innovated in the EP space in both imaging and devices for many years already, as Philips has developed cutting-edge interventional systems, software and lead management solutions. Now through our EPD Solutions business, we are bringing EP procedures to a new level, with a solution set to transform the EP field. The KODEX-EPD system uses dielectric imaging rather than x-ray, to create 3D CT-like, high-definition images of a patient’s cardiac structures in real time, simplifying navigation and improving efficiency, without using radiation. Considering that electrophysiologists and their staff perform many procedures a day, this is a big deal! To advance this breakthrough innovation, we recently announced a collaboration with Medtronic to combine their cryoablation solution with our KODEX-EPD platform.
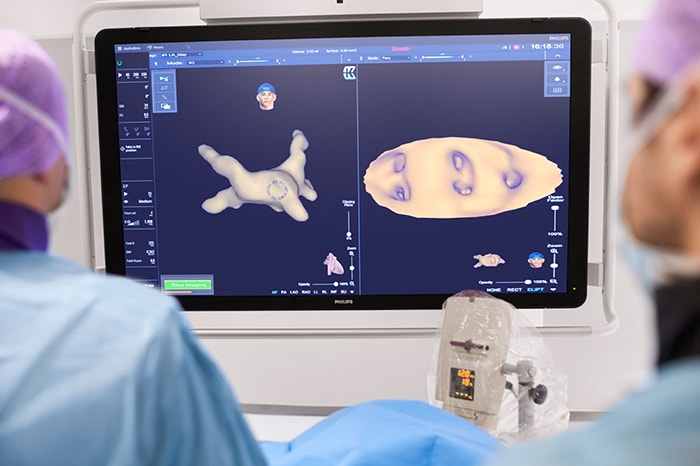
Philips’ KODEX-EPD platform uses dielectric imaging to create high-definition 3D images of a patient’s cardiac structures, in real time
But that’s just the start. In the future this technology has real potential to improve AFib treatment worldwide. Right now, a large number of patients – remember, up to 40% – who undergo AFib ablation have the condition return and face a second round of treatment. In the future, this new solution could tackle our holy grail of real-time ablation assessment, allowing treatment teams to confirm during treatment that they got it ‘first-time right’. From wearables, to imaging systems, devices, and soon KODEX-EPD, physicians will have more tools than ever to help us decide, guide, treat and confirm AFib treatment. The future is bright for AFib patients and I am excited to see where technology takes us next.
Share on social media
Topics
Author

Atul Gupta
Chief Medical Officer, Image Guided Therapy Atul Gupta, MD is Chief Medical Officer at Philips’ Image Guided Therapy and a practicing interventional radiologist. Prior to joining Philips in 2016, Atul served on Philips’ International Medical Advisory Board for more than 10 years. Atul continues to perform both interventional and diagnostic radiology in suburban Philadelphia, in both hospital and office-based lab settings. He has been repeatedly recognized as top physician for his specialty in the media and serves on several advisory boards. He has also published and lectured internationally on a range of interventional procedures.
Follow me on