Philips Chief Innovation Officer Shez Partovi comments on navigating the EU AI Act
May 13, 2025 | 2 minute read
As a global leader in health technology, Philips believes that the regulatory framework for and the regulation of AI medical devices must ensure the safety of patients, while allowing the continuation of a thriving and competitive European AI ecosystem. The implementation of the EU AI Act should not result in additional certification costs, the delay of product launches, over-regulation and unnecessary obligations that could hamper AI adoption and innovation in Europe.

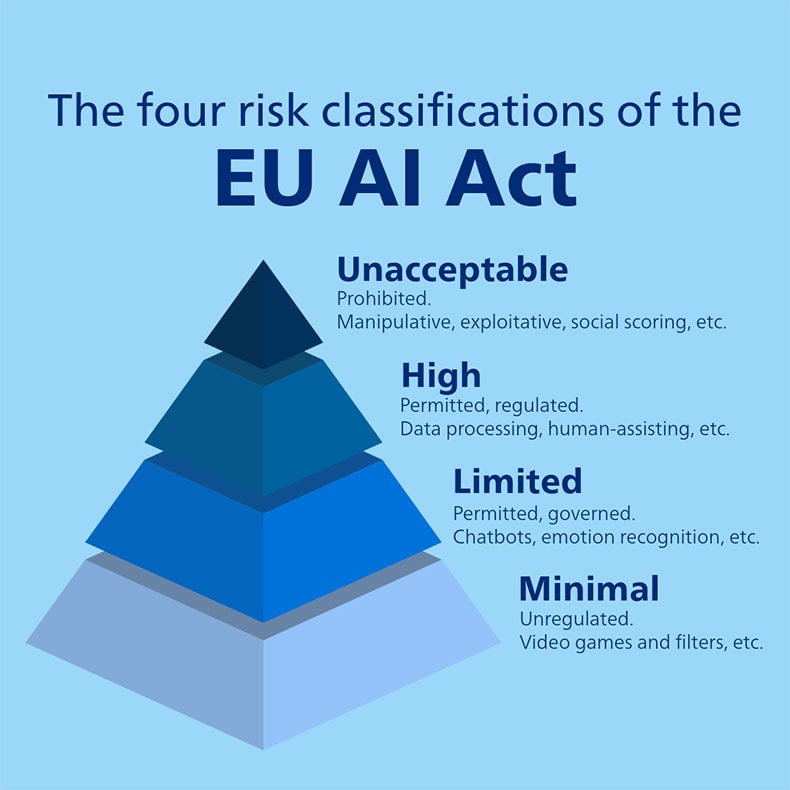
The AI Act, a European regulation on artificial intelligence (AI), assigns applications and systems to four risk categories [1]. The regulation became law last summer and will be fully applicable to high-risk AI systems – embedded into regulated products – by August 2027, namely any medical devices with AI applications.
A recent issue of PoliticoPro’s Morning Health Care Newsletter reports that, under the AI Act, many AI medical devices will likely be classified as high-risk, meaning they will be subject to a series of strict compliance obligations. Medical devices with AI components will need to comply with both the Medical Devices Regulation (MDR) and the AI Act. Notified bodies will need to be designated to assess products under the AI Act, similar to their designation under MDR. However, not all notified bodies assessing medical devices may choose to be designated for AI medical devices.
Politico highlights how notified bodies are preparing to assess AI medical devices under the AI Act and how countries will have to designate national competent authorities by August 2025, who will then inspect and designate notified bodies. Manufacturers, including Philips, hope to be able to apply to one notified body for compliance checks under both regulations. And while many AI Act requirements overlap with MDR, there are additional requirements under the EU AI Act.
The article further explains how manufacturers are concerned that overlapping requirements will stifle innovation and includes quotes by Shez Partovi, Chief Innovation Officer at Philips.
“Companies like Philips may actually be positioned to overcome that headwind because of our size and scale, but young companies, startups, forget it,” he told Claudia [Chiappa, Health Reporter at Politico Europe].
Gearing up for the change: Partovi said they have been preparing for the moment the AI Act enters into full force, training their employees on the requirements of the AI Act. But it’s a “Herculean task.”
“There’s still hope that we will adjust and correct the course, but our position as a global company based in Europe is that we want to make sure that trust is built, but not burdened in a way that we can’t bring innovation as fast. I mean, it would be a shame to bring innovation faster in the North America market than in the European market for a European company.”








