DEFINE GPS trial marks major milestone in interventional cardiology
May 13, 2025 | 6 minute read
Philips and its clinical partners have reached a major milestone in the landmark DEFINE GPS clinical trial—a global, randomized controlled study evaluating whether angiography combined with iFR (instantaneous wave-free ratio) co-registration can improve outcomes for patients undergoing percutaneous coronary intervention (PCI).

As part of its long-standing commitment to evidence-based innovation, Philips works closely with clinical partners worldwide to co-develop and validate technologies that support better outcomes, improved workflows, and more personalized, cost-effective care. DEFINE GPS is a prime example of this approach in action. With nearly 2,200 coronary artery disease patients enrolled across 85 hospitals in 18 countries, DEFINE GPS is one of the largest and most comprehensive studies of its kind—integrating physiological guidance and imaging to assess procedural effectiveness.
We spoke with Dr. Darshan Doshi, Head of Medical & Clinical at Philips Image-Guided Therapy Devices, about why this study is so important, how it could reshape interventional cardiology, and what it means for patients and providers alike.

Dr. Darshan Doshi, Head of Medical & Clinical at Philips Image-Guided Therapy Devices
Q: DEFINE GPS has just completed enrollment—close to 2,200 patients. What does that moment mean to you and the team?
It’s a tremendous achievement! We randomized over 2,182 patients across 18 countries and 85 global sites. That level of coordination—among investigators, clinical research coordinators, and our Philips team—is extraordinary. This is a true global effort, with participating countries including the U.S., Australia, Canada, Israel, Germany, the Netherlands, and many more. Completing enrollment is a major milestone.
Q: Can you walk us through the journey of getting to this point?
From the start, this was a very ambitious trial. Educating clinicians on the value of physiology and training them to use iFR co-registration effectively was a huge lift. But we had a clear goal: to answer whether using iFR co-registration leads to better outcomes than angiographic guidance alone during PCI. This trial, which was conceived more than five years ago, enrolled its first patient in 2021 and getting to this point has required dedication and coordination across multiple continents.
Q: For those unfamiliar, what exactly is DEFINE GPS studying?
PCI is a minimally invasive procedure that is widely used for the treatment of coronary artery disease. The DEFINE GPS trial is a prospective, randomized controlled trial comparing traditional PCI guided by angiography to PCI guided by angiography combined with IFR co-registration using Philips’ pressure guidewire and SyncVision system. We’re looking at clinical efficacy, safety, quality of life, and economic outcomes.
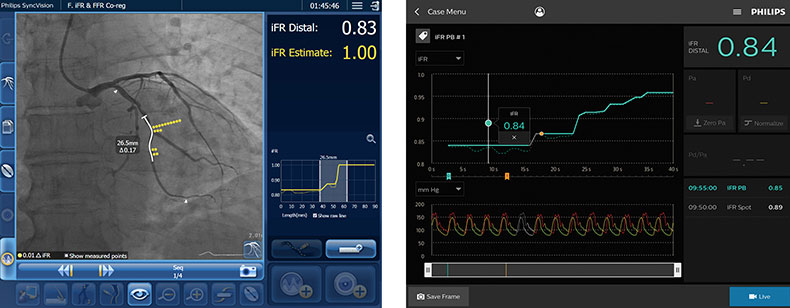
Q: How does IFR co-registration work, and how is it different from angiographic guidance?
With conventional angiography, you inject contrast to image the coronary arteries and visually assess blockages. But the appearance of a blockage doesn’t always reflect physiological impact on the blood across the blockage. IFR co-registration adds a pressure wire that measures where pressure drops in the coronary arteries—those areas may be causing symptoms or carry higher risk. It helps tailor treatment by identifying which blockages actually need stent treatment and confirms whether the treatment was effective.
Q: Why is this trial important now for interventional cardiology? The opinions and clinical experiences presented herein are specific to the featured topics and are not linked to any specific patient and are for information purposes only. The medical experience(s) derived from these topics may not be predictive of all patients. Individual results may vary depending on a variety of patient-specific attributes and related factors. Nothing in this article is intended to provide specific medical advice or to take the place of written law or regulations
We always strive for better outcomes—lower mortality, fewer heart attacks, and less re-hospitalization. This trial could help us achieve that. There’s also a cost element: by treating patients more precisely, we could reduce unnecessary procedures and hospital visits. It’s about delivering better care for more people while potentially easing the economic burden on healthcare systems.
Q: Many patients still experience chest pain after PCI. How could this study help address that?
In our earlier DEFINE PCI study, we found that 1 in 4 patients left the cath lab with residual ischemia, even after treatment. In 80% of those cases, additional stenting or balloon dilation would’ve resolved the issue. DEFINE GPS builds on that by using IFR co-registration to guide treatment more precisely—and verify success before the patient leaves the table.
Q: What are the main outcomes you’ll be analyzing over the next two years?
The primary endpoint is the rate of major adverse cardiac events, which is a composite of cardiac death, myocardial infarction, and ischemia-driven revascularization, or hospitalization for progressive or unstable angina at 2-years. We’re also measuring quality of life, resource utilization, and cost-effectiveness, all tracked at 30-days, one year, and two years.
Q: If the results are positive, how could this change how we define a successful PCI?
This could represent a paradigm shift in how we approach PCI. We may begin to routinely incorporate physiologic confirmation, using iFR, to verify treatment effectiveness before concluding the procedure. If the data validates this strategy, it has the potential to influence international clinical guidelines.
Q: And how could IFR co-registration improve decision-making for physicians in real time?
It brings objectivity. Say a physician sees two or three blockages—one might appear severe; the others may appear borderline on the angiogram. IFR co-registration tells you which ones are actually significant and helps you map out your stenting strategy. And after treatment, it confirms successful treatment and helps ensure no key steps are missed.
Q: Does this make PCI more consistent and precise, especially in complex cases?
Yes. Not only is the assessment of blockages difficult, one of the biggest challenges in PCI guided by angiography alone is also the variability between operators. What looks like a 70% blockage to one doctor may look like 40% to another. IFR co-registration removes that subjectivity and brings more standardization, especially in borderline or multi-vessel disease cases.
Q: With enrollment completed, what’s next for the DEFINE GPS teams across the world?
Now we enter the follow-up and analysis phase. The primary endpoint is at two years, so we’re focused on thorough clinical follow-up. That includes reviewing medical records and capturing any endpoints like myocardial infarction, revascularization, or death. The more comprehensive the data, the more robust our conclusions will be.
Q: Do you expect the results to influence global clinical practice guidelines?
That’s the ambition of the trial. IFR already has a Class 1A recommendation for invasive physiology in PCI—no other non-hyperemic index does. If we show that layering IFR co-registration on top of that improves outcomes, it would make a strong case for updating guidelines worldwide.
Q: How does this trial reflect Philips’ approach to innovation?
DEFINE GPS is one of the largest clinical trials that Philips has ever sponsored. It reflects our conviction that innovation must be evidence-based. Whether it’s cardiology or other areas of care, our goal is to bring the best tools, practices, and technologies to clinicians—and provide better care for more people.