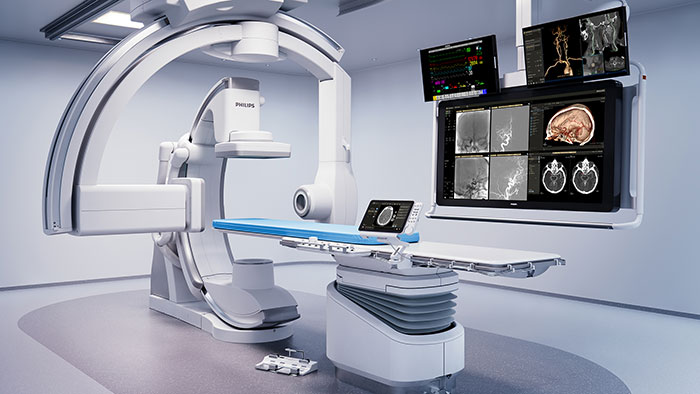
Philips and Polarean partner to bring advanced Xenon lung MRI to more children with chronic obstructive lung disease
New MRI technology transforms pulmonary imaging to help doctors see how air moves in the lungs, offering a safer, clearer view of lung function for children as young as 6, pending FDA approval [1]
May 14, 2025 | 3 minute read
Philips and Polarean, a medical imaging technology leader in advanced MRI of lung function, today announced the next phase of their strategic partnership to increase access to advanced, radiation-free lung MRI for patients with obstructive lung diseases. By expanding multi-nuclei imaging with Xenon MRI, the collaboration enables clinicians to better detect and monitor lung conditions like asthma and cystic fibrosis. Unlike standard imaging, this new technique, using hyperpolarized Xenon gas as a signaling agent, allows clinicians to see how air flows through different parts of the lungs in real time, providing critical insight that was previously invisible. Xenon MRI is already FDA-approved for evaluating lung ventilation in adults and children aged 12 and older. Pending FDA approval for children as young as six, this collaboration is a major step toward earlier detection and safer, long-term disease monitoring, without the risks associated with radiation exposure.
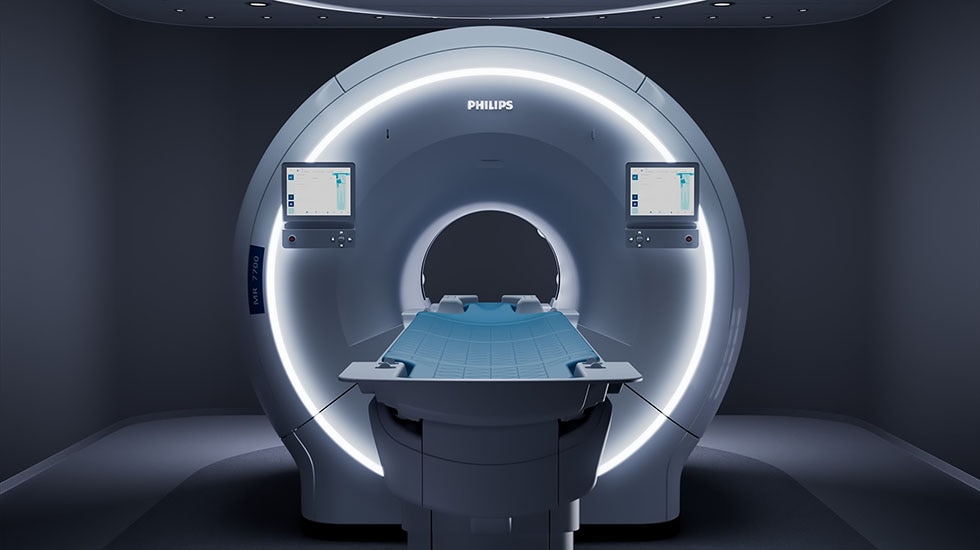
Obstructive lung diseases like asthma and COPD affect over 42 million people in the United States and more than 500 million worldwide, placing a major burden on global respiratory health [2]. Yet common imaging techniques currently used often lack the detail to show how these conditions affect lung function. There is a clear unmet need for safe, accurate, and non-invasive imaging tools to visualize lung ventilation without radiation exposure. This is particularly urgent in pediatrics, where repeat imaging is often necessary to track disease progression or treatment response.
Philips and Polarean are addressing this need by bringing advanced, non-invasive Xenon MRI into routine practice and increasing access to younger patients. Now integrated into Philips' clinical workflow across 3.0T MRI systems, with just two 10-second breath-holds [3], the scan session can be completed in under a minute, using the FDA-cleared Polarean XENOVIEW 3T Chest Coil [4]. This allows clinicians to switch seamlessly between Xenon and standard Proton imaging, without moving the patient or changing coils, making it easier for both patients and imaging teams. For the first time, clinicians can visualize regional lung ventilation directly, enabling more accurate disease detection and monitoring.
By combining Philips’ MRI platform with Polarean’s Xenon MRI technology, we can now routinely and efficiently visualize how air moves through a child’s lungs, without the risks of radiation.
"Our mission is to pioneer advanced imaging techniques that make a real difference in the lives of children with chronic lung disease. By combining Philips’ MRI platform with Polarean’s Xenon MRI technology, we can now routinely and efficiently visualize how air moves through a child’s lungs, without the risks of radiation. This solution transforms our ability to deliver precise, functional insights right at the bedside, accelerating the path from innovation to impact. It’s an incredible step forward, and I’m honored to be part of work that’s changing how we care for young patients," said Dr. Laura Walkup, Associate Professor, Divison of Pulmonary Medicine at Cincinnati Children’s Hospital Medical Center.
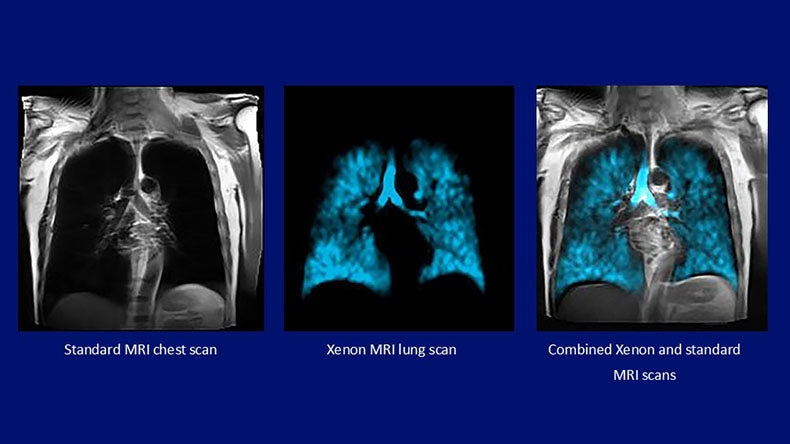
“By integrating the Xenon MRI platform into our advanced 3.0T MRI systems, we’re delivering a powerful, non-invasive tool that can transform how we diagnose, monitor, and manage chronic lung disease,” said Dr. Ioannis Panagiotelis, Business Leader of MRI at Philips. “This radiation-free technology is especially important for younger patients and has the potential to shift multi-nuclei imaging from research to everyday clinical care. We’re proud to be leading the way in making this innovation a routine part of clinical care.”
“Using our FDA-cleared Polarean XENOVIEW 3T Chest Coil, clinicians can perform both Xenon and Proton scans during two 10-second breath-holds without needing to reposition the patient or switch coils. The procedure is intuitive via the Philips user interface and keeps total in-bore time to around one minute,” said Dr. Christopher von Jako, Chief Executive Officer at Polarean. “Philips and Polarean share a common goal: to make lung imaging more precise, accessible, and patient-friendly. The introduction of the XENOVIEW 3T Chest Coil, combined with Philips’ robust MRI platforms, marks a significant step in realizing that vision.”
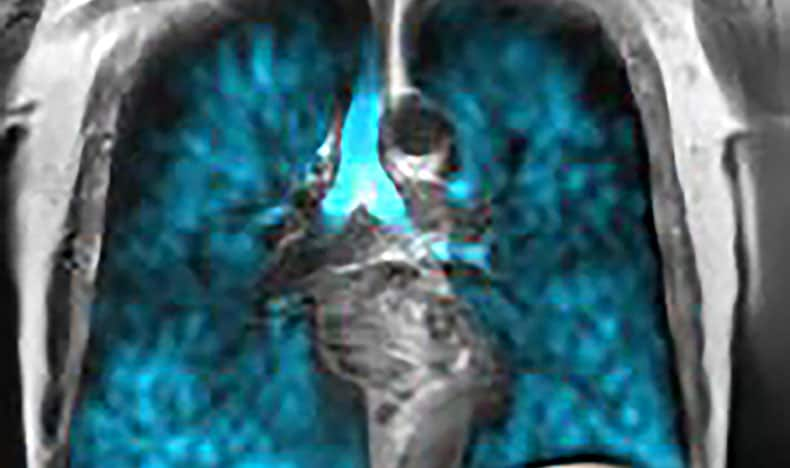
Philips and Polarean to showcase next-generation lung imaging at #ISMRM2025
At the upcoming ISMRM Annual Meeting, Philips and Polarean will highlight their latest advancements in multi-nuclei MRI, including high-resolution, non-invasive imaging of regional lung ventilation. These innovations expand access to advanced pulmonary imaging for patients. Since launching its integrated multi-nuclei imaging solution in 2022, Philips has seen global traction, with 1 in 10 3.0T MRI sites now using non-proton nuclei clinically, driven in part by its partnership with Polarean and the Xenon MRI platform. Philips remains the only vendor with multi-nuclei imaging fully integrated into clinical workflows.
Sources [1] Polarean submitted a Supplemental New Drug Application (sNDA) to the US Food and Drug Administration (FDA) last July, seeking to expand its current lung ventilation MRI indication by lowering the minimum patient age from 12 years to include pediatric patients as young as six.
[2] https://www.who.int/news-room/fact-sheets/detail/chronic-obstructive-pulmonary-disease-(copd)#:~:text=Key%20facts,Symptoms
[3] https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=214375
[4] Integration of the FDA-cleared Polarean XENOVIEW Chest coil with Philips 3.0T MRI systems is a work in progress. The XENOVIEW chest coil is not to be used yet on pediatric patients younger than 12 years.