Prostate Cancer Diagnosis: connecting radiology and urology workflows for better care
May 23, 2025 | 3 minute read
With prostate cancer being the 4th most common cancer worldwide and the 2nd most common cancer among men [1], early and accurate diagnosis is as crucial as ever, as it often leads to clear options for treatment. The standard way to diagnose is often still a transrectal ultrasound (TRUS) prostate biopsy, which can be imprecise, with the ability to detect only 31-40% of cancers [2].
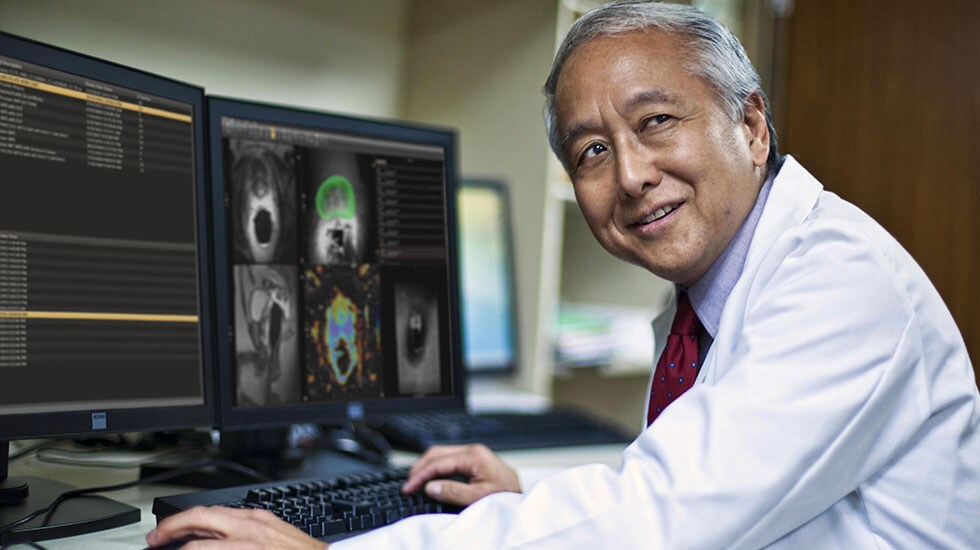
Over the past years, multi-parametric Magnetic Resonance Imaging (mpMRI) has gained prominence as a way of obtaining a more targeted and accurate biopsy. Based on MR images of the prostate, the radiologist can define suspicious areas and provide a score (PI-RADS) which rates the likelihood of cancer in an abnormal area or lesion. Fusing these MR images with TRUS images in real time allows the urologist to see suspicious lesions while visualizing the biopsy needle at the same time leveraging both ultrasound and MR images – offering more precise guidance for biopsy-taking. This more targeted approach can benefit patients by potentially reducing instances of biopsy-related hospitalization, decreasing the likelihood of sepsis, and most importantly, avoiding the need for repeat biopsies [3].
Using MR imaging and automated advanced post-processing can also enhance workflow, productivity and collaboration between radiology and urology departments and clinicians. Philips’ prostate cancer care solutions include DynaCAD, an advanced visualization system designed to enhance the workflow of radiologists and UroNav, an advanced fusion biopsy system that supports prostate biopsies by fusing pre-biopsy MR images with live ultrasound images for guided biopsies in real time. By integrating advanced visualization and fusion biopsy technologies, patients receive better and more precise care and clinicians are supported in better diagnosis, a 30% improvement in high-risk prostate cancer diagnosis using fusion biopsy compared to the standard biopsy [4].
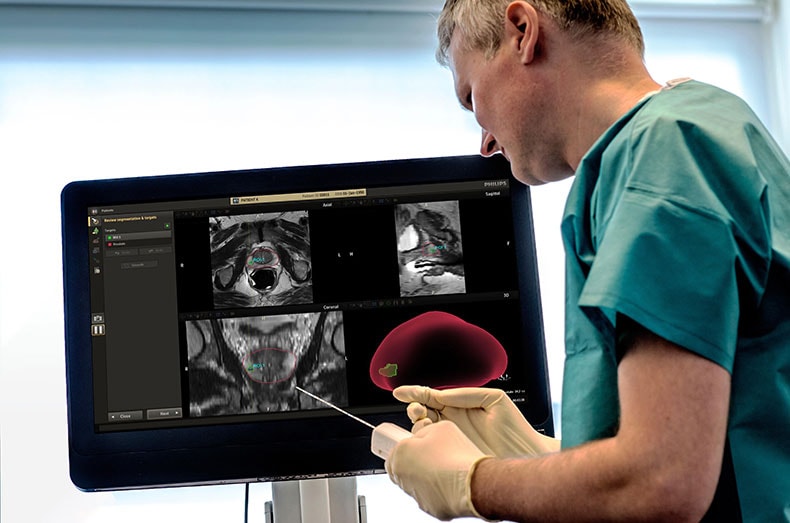
This integrated approach ensures that the prostate segmentation, identification, and segmentation of prostate cancer lesions, are transferred from DynaCAD to UroNav. Meaning that the radiologist’s results can be shared with the urologist, who can then use this information to prepare and execute the biopsy procedure.
As João Magalhães Pina, MD, Urologist, Hospital da Luz, Lisboa, Portugal said: “A competitive advantage of the Philips UroNav fusion biopsy device is that it is fully integrated with DynaCAD Prostate software: a urologist does not have to perform the contouring, which is the outlining or marking, of the prostate and lesions on MR images, as it is performed beforehand by a radiologist with the DynaCAD Prostate software. Ready-to-use data are transferred from radiology to urology, making it easier for the urologist to target the lesions during biopsy procedure.”
The most recent version of Philips DynaCAD 5.1 has received its EU Medical Device Regulation (MDR) certificate, including optimized MR image reading and automated lesion segmentation, which means clinicians have even quicker and more efficient access to crucial diagnostic data.
By enhancing collaboration, boosting productivity, and streamlining workflows between radiology and urology, caregivers can confidently provide their patients with the precise care they deserve.
“With these integrated solutions, we streamline the path to precise diagnosis for prostate cancer providing radiologists and urologists with the information and tools to efficiently and precisely locate, score, and target lesions with fusion biopsy, connecting the data across specialties," said Martijn Hartjes, Business Leader Clinical Informatics at Philips. "By enhancing collaboration, boosting productivity, and streamlining workflows between radiology and urology, caregivers can confidently provide their patients with the precise care they deserve.”
Philips comprehensive portfolio ensures precise and efficient prostate cancer diagnosis and treatment, leveraging advanced imaging, biopsy guidance, and digital pathology solutions. Philips’ digital pathology solution includes whole-slide scanners, image management system, and proven implementation and support services to guide the transformation from analog to fully digital pathology workflows. By expanding access and streamlining the use of AI-powered tools in digital pathology, labs will become more efficient and effective, addressing the pressure of a global shortage of pathologists and the growing number of cancer patients.
Sources [1] World Cancer Research Fund, link: https://www.wcrf.org/preventing-cancer/cancer-statistics/prostate-cancer-statistics.
[2] Choi YH, et al. Comparison of cancer detection rates between TRUS-guided biopsy and MRI-targeted biopsy according to PSA level in biopsy-naive patients: a propensity score matching analysis. Clin Genitourin Cancer. 2019 Feb;17(1):e19-e25. doi: 10.1016/j.clgc.2018.09.007. Epub 2018 Sep 13. PMID: 30415878.
[3] Sung H, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021.71:209-249. https://doi.org/10.3322/caac.21660
[4] Siddiqui MM, et al. Comparison of MR/ultrasound fusion–guided biopsy with ultrasound-guided biopsy for the diagnosis of prostate cancer. JAMA. 2015;313(4):390-397.




