Nov 02, 2021
TCT 2021: Philips announces new innovations and clinical data supporting the treatment of patients with cardiovascular disease
Amsterdam, the Netherlands – Royal Philips (NYSE: PHG, AEX: PHIA), a global leader in health technology, today announced North American availability of new innovations in its portfolio of cardiology solutions designed to seamlessly work together, strengthen clinical confidence, build efficiency throughout the care pathway, and improve cardiac care outcomes and experiences. The new updates, aimed at innovating and advancing procedures including percutaneous coronary intervention (PCI) to treat the narrowing of coronary arteries, are being announced at the Transcatheter Cardiovascular Therapeutics (TCT) annual meeting (Orlando, USA, November 4-6). In addition, Philips is putting a spotlight on new clinical data being presented at TCT that shows how novel minimally-invasive techniques are helping to innovate interventional procedures and improve patient outcomes. The demands on cardiology departments are higher than ever before, forcing clinicians to balance the delivery of high-quality care for a growing volume of complex patients with the challenge of improving departmental efficiency at the same time.
At this year’s TCT, we’re showing how we are continuously innovating and expanding our integrated ecosystem of interventional imaging systems and diagnostic and therapeutic devices to provide clinicians with a complete solution, from diagnosis to treatment and therapy monitoring, to optimize their workflow and the treatment of each individual patient.
Chris Landon
Senior Vice President and General Manager, Image Guided Therapy at Philips
“Interventional physicians seek ever-more precise, accurate and efficient tools to decide, guide, treat and confirm the right therapy for the right patient at the point of care,” said Chris Landon, Senior Vice President and General Manager, Image Guided Therapy Devices at Philips. “At this year’s TCT, we’re showing how we are continuously innovating and expanding our integrated ecosystem of interventional imaging systems and diagnostic and therapeutic devices to provide clinicians with a complete solution, from diagnosis to treatment and therapy monitoring, to optimize their workflow and the treatment of each individual patient.”
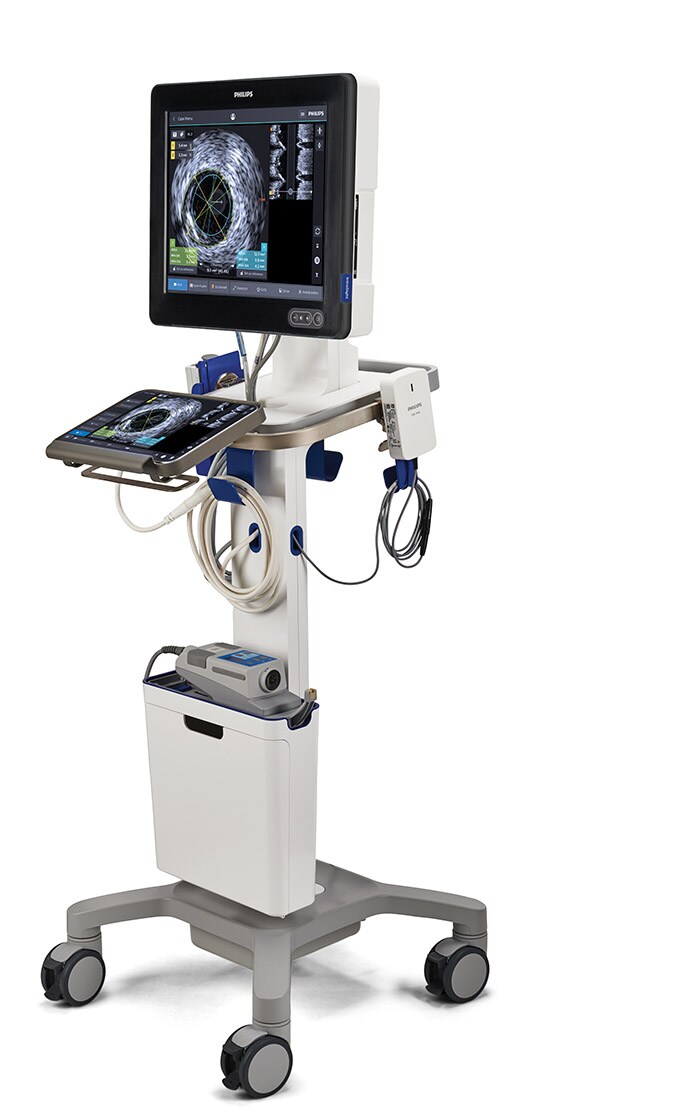
North America debut of Philips’ mobile interventional applications platform
The new Philips Interventional Applications Platform – IntraSight Mobile – is now available in North America. IntraSight Mobile brings together imaging and physiology applications on a mobile system for coronary and peripheral artery disease therapy. The IntraSight platform allows interventional cardiologists to perform intravascular ultrasound (IVUS) imaging and physiologic measurements of fractional flow reserve (FFR) and instant wave-free ratio (iFR) to accurately identify the location of lesions causing ischemia. IntraSight Mobile builds on the success of Philips Interventional Applications Platform – IntraSight – a solution that integrates intravascular diagnostic applications into a smart, simple and seamless workflow solution for interventional labs. IntraSight Mobile is compatible with Philips’ current and future portfolio of imaging and physiology modalities. The system includes a touchscreen panel PC and a ruggedized, multi-modality touchscreen module mounted on a modern and easy to maneuver mobile cart for all environments. IntraSight Mobile offers clinicians an intuitive user interface and simplified workflow, enhancing the user experience while optimizing lab performance. Clinicians can customize the platform and select best-in-class tools that are right for their coronary or peripheral vascular patients.
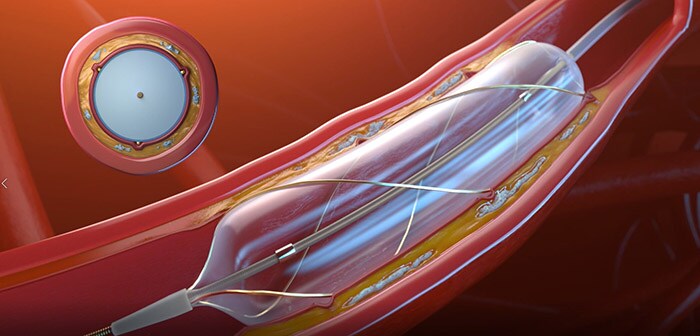
Superior deliverability with new scoring balloon
The newly designed Philips Scoring Balloon Catheter RX – AngioSculpt Evo – has superior deliverability and the power to safely dilate resistant lesions [1, 2, 3]. It comes with a combination of changes, including a smaller tip for smoother lesion entry, a hydrophilic coating to reduce friction, and a laser-cut hypotube for enhanced flexibility. The helical nitinol scoring elements wrap the balloon around its circumference to minimize slippage and lock to the lesion, delivering up to 25 times [1] the force of a non-compliant balloon. This device is available for treatment of coronary artery stenosis, including in-stent restenosis.
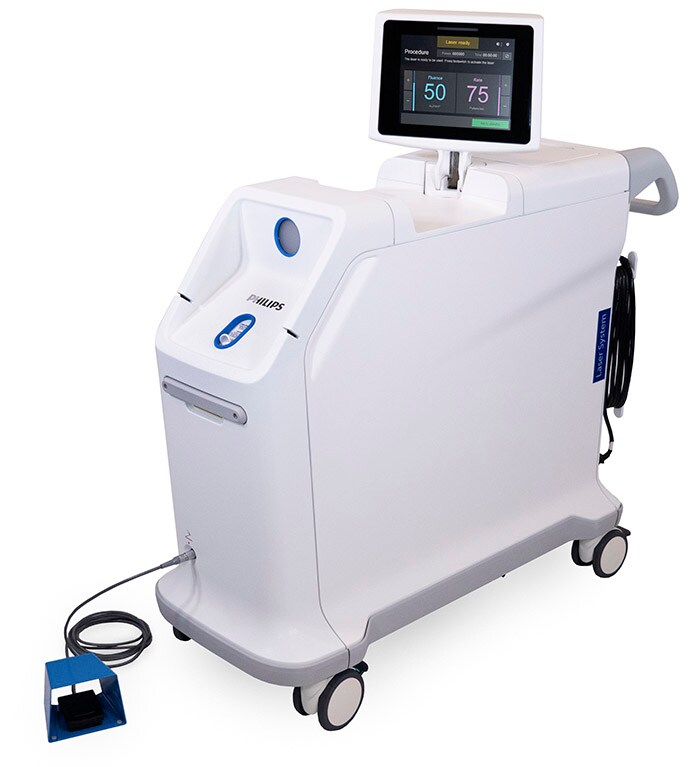
Introduction of new laser system
The new Philips Laser System – Nexcimer – offers plug-and-play simplicity for coronary and peripheral atherectomy and lead extraction procedures. Smaller, lighter, and more maneuverable than the previous generation [4], the system starts up within 30 seconds, uses standard medical-grade 100-240V outlets, and has an intuitive touchscreen interface with guided workflow prompts that help lessen the need for technician training. It is the only system compatible with catheters with Level I clinical data for ISR atherectomy and that can also support lead extraction procedures (the removal of pacemaker or defibrillator leads around the heart) [5, 6].
An innovative cardiology experience with Philips at TCT 2021
Visitors to TCT 2021 will have access to Philips’ latest solutions, plus opportunities to engage with clinical experts and experience innovative cardiac solutions. Philips’ technology advancements in diagnostic, interventional and monitoring solutions at TCT 2021 include:
New clinical data presented at TCT 2021
Five-year outcomes of the iFR-SWEDEHEART trial: On Thursday, November 4, at 1:45pm EST/7:45pm CET, as part of TCT’s Late-Breaking Clinical Trials and Science program, five-year outcomes of the iFR-SWEDEHEART trial will be presented by Matthias Götberg, MD, PhD, interventional cardiologist and Director of the Cardiac Catheterization Lab at Skane University Hospital, Lund, Sweden. Join to hear the data results: iFR-SWEDEHEART: Five-Year Outcomes of a Randomized Trial of iFR-Guided vs. FFR-Guided PC.
Large-scale study outcomes on use of IVUS: On Saturday, November 6, at 2:24pm EST/8:24pm CET, Eric A. Secemsky, MD, Interventional Cardiologist at Beth Israel Deaconess Medical Center and Assistant Professor of Medicine at Harvard Medical School in Boston, MA, will present a new large-scale analysis of Centers for Medicare & Medicaid Services (CMS) data on the clinical and economic outcomes of diagnosing and treating peripheral vascular disease using intravascular ultrasound (IVUS). Learn more here about his TCT Innovation Theater session titled: Endovascular Featured Clinical Research – Utilization of Intravascular Ultrasound During Peripheral Venous Intervention Among Medicare Beneficiaries.
Philips hosted webinars, symposium, and virtual cardiology experience
As the patient population increases in age so does the presentation of high-risk individuals with multiple co-morbidities. On Thursday, November 4, at 4:00pm EST/10:00pm CET, tune in live at TCT to hear from Dr. Ziad A. Ali, Dr. Allen Jeremias, and Dr. Karim Al-Azizi on how they perform ultra-low contrast PCI to treat their high-risk patients and learn what tools and techniques they adopt to optimize patient outcomes. Refer to full Philips Laser System device labeling and instructions for important safety information. Caution: Federal law restricts this device to sale by or on the order of a physician. [1] D051336 AngioSculpt Evo Marketing Claims Report. Internal AngioSculpt Test Report SR-1571.A (2008).
For the full calendar of events, as well as general information about Philips’ presence at the show, visit www.philips.com/TCT. Visit the Philips Industry Hub to experience innovative cardiac solutions and follow the #TCT2021 conversation on @PhilipsLiveFrom throughout the event.
[2] Costa RA, Mooney MR, Teirstein PS, et al. Final results from the multi-center trial of the angiosculpt scoring balloon catheter for the treatment of complex coronary artery lesions Cardiovascular Revascularization Medicine 7 (2006)81–126.
[3] Costa JR, Mintz GS, Carlier SG, et al. Nonrandomized comparison of coronary stenting under intravascular ultrasound guidance of direct stenting without predilation versus conventional predilation with a semi-compliant balloon versus predilation with a new scoring balloon. Am J Cardiol. 2007;100:812-817.
[4] Comparison to previous generation (CVX-300).
[5] Philips Laser System Data Sheet Specifications.
[6] Dippel et al. Randomized Controlled Study of Excimer Laser Atherectomy for Treatment of Femoropopliteal In-stent Restenosis: Initial ISR Results (2015). JACC 8(1): 92-101.
[7] Gotberg M, et al., iFR-SWEDEHEART Investigators. Instantaneous Wave-free Ratio versus Fractional Flow Reserve to Guide PCI. N Engl J Med. 2017 May 11;376(19):1813-18233.
About Royal Philips
Royal Philips (NYSE: PHG, AEX: PHIA) is a leading health technology company focused on improving people's health and well-being, and enabling better outcomes across the health continuum – from healthy living and prevention, to diagnosis, treatment and home care. Philips leverages advanced technology and deep clinical and consumer insights to deliver integrated solutions. Headquartered in the Netherlands, the company is a leader in diagnostic imaging, image-guided therapy, patient monitoring and health informatics, as well as in consumer health and home care. Philips generated 2020 sales of EUR 17.3 billion and employs approximately 78,000 employees with sales and services in more than 100 countries. News about Philips can be found at www.philips.com/newscenter.
Topics
Contacts

Joost Maltha
Philips Global Press Office Tel: +31 6 10 55 8116
You are about to visit a Philips global content page
Continue
Fabienne van der Feer
Philips Image Guided Therapy Tel: + 31 622 698 001
You are about to visit a Philips global content page
ContinueMedia assets
Press releases
Get our press releases by e-mail
You are about to visit a Philips global content page
Continue