Philips teams with Synthetic MR to deliver breakthrough AI-based quantitative brain imaging in MR to advance neurology care for patients at #ECR2024
Feb 29, 2024 | 3 minute read
Amsterdam, the Netherlands and Vienna, Austria – Royal Philips (NYSE: PHG, AEX: PHIA), a global leader in health technology, and magnetic resonance imaging (MRI) software solutions company Synthetic MR (Linköping, Sweden) today announced the launch of Smart Quant Neuro 3D [1] – a major advance in objective decision support for diagnosis and therapy assessment of brain disorders like multiple sclerosis (MS), traumatic brain injury (TBI), and dementia. As healthcare providers strive to deliver high-quality care to patients, at #ECR2024 Philips is partnering with its customers to improve productivity and access to more sustainable healthcare.
Smart Quant Neuro 3D combines Philips’ AI based SmartSpeed image-reconstruction technology, Philips 3D SyntAc clinical application and Synthetic MR’s SyMRI NEURO 3D [2] quantitative tissue assessment software. The combined offering gives healthcare providers powerful tools for greater diagnostic confidence ultimately benefiting patients. Philips’ exclusive agreement with SyntheticMR makes it the only company currently able to offer SyMRI NEURO 3D capability on MR scanners.
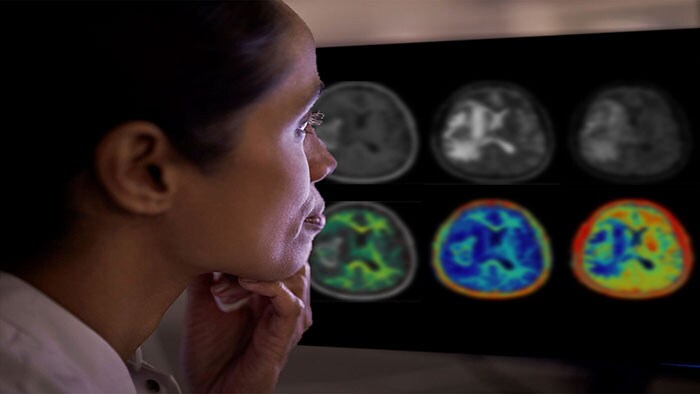
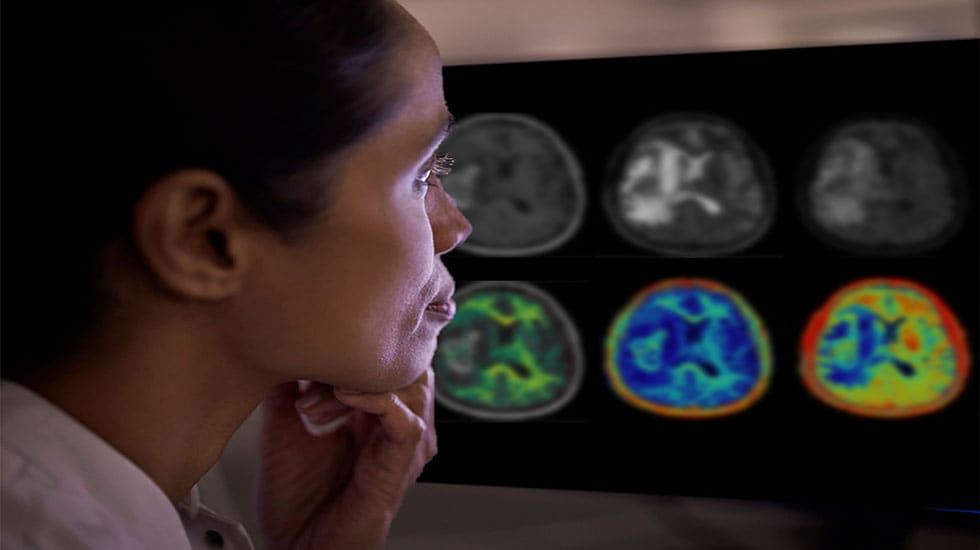
Accurate measurement of the volume and distribution of different tissue types in the brain is important for diagnosing brain disease. However, visual assessments of MR images are often subjective, and lack the necessary accuracy while measuring tissue volume from 2D image slices is virtually impossible. Smart Quant Neuro 3D leverages the power of AI to provide fully verified automatic and precise 3D segmentation and volume measurement of brain tissue such as white matter, gray matter, cerebrospinal fluid, and myelin. When interpreted by a trained physician, these measurements can provide useful information in determining diagnosis for patients.
“Life-changing brain injury and neurodegenerative disease are two of the most difficult diagnoses that clinicians have to make on a daily basis, because of the different symptoms exhibited by individual patients”, said Ruud Zwerink, Business Leader of MR at Philips. With Smart Quant Neuro 3D, clinicians have access to an easy-to-use tool to provide valuable quantitative data to track the impact of treatments and make informed decisions about adjustments or alternative interventions as needed. This continuous monitoring enhances patient care by enabling proactive management of neurological conditions and optimizing therapeutic outcomes for patients.
Smart Quant allows for shorter exams and exploration of quantitative metrics, which show great potential to better stratify patients with similar imaging characteristics on conventional MR sequences.
“Smart Quant allows for shorter exams and exploration of quantitative metrics, which show great potential to better stratify patients with similar imaging characteristics on conventional MR sequences. It’s a fast quantitative MR technique that’s integrated into our clinical workflow, allowing us to generate multiple synthetic weighted images from a single sequence,” said Dr. Julien Savatovsky, Neuroradiologist and Head of the Radiology Department at the Hospital Foundation Adolphe De Rothschild (Paris, France).
Enhanced diagnostic confidence to help improve treatment strategies for patients
Smart Quant Neuro 3D is particularly effective at measuring myelin, the insulating layer around nerves that ensures electrical impulses in the brain reach their destination. Loss of myelin is a major characteristic of TBI, where Smart Quant Neuro 3D’s ability to detect abnormalities that today remain invisible on conventional MR scans, helps with early diagnosis and intervention. By quantitatively measuring myelin, clinicians can better understand the extent and impact of TBI and differentiate TBI from other neurological diseases.
The ability to quantify the extent of myelin loss or damage in a patient’s brain is also important in progressive brain diseases such as multiple sclerosis. Quantitative measurement of myelin loss allows clinicians to assess the condition, track its progression over time, and evaluate the effect of the latest disease-modifying drugs. The additional information delivered by Smart Quant Neuro 3D benefits patients since all relevant clinical MR imaging contrasts are available from a single acquisition, avoiding the need for patients to be recalled to collect additional data.
Improved workflow efficiency for clinicians
Leveraging Philips’ AI-based MR SmartSpeed reconstruction technology, which increases imaging speed by a factor of nearly 3 times while providing up to 65% greater resolution [3], Smart Quant Neuro 3D assists in the scanning of patients and can help to accelerate radiology department throughput. SyntheticMR’s SyMRI NEURO 3D software then performs an automatic (zero-click) analysis of the imaging data to provide accurate segmentation and volume measurement of tissue in less than 10 seconds of post-processing.
Sources [1] Philips 3D SyntAc used in combination with SmartSpeed provides the input for the Synthetic MR software, SyMRI. Any quantification reference in the present material is provided soley by SyMRI software.
[2] Smart Quant refers to the compatibility between the cleared features SmartSpeed (AI enabled) with the quantitative imaging technique [SyntAc]. Smart Quant Neuro 3D is a combination of Philips SmartSpeed and Philips 3D SyntAc and Synthetic MR’s SyMRI. Philips 3D SyntAc is FDA approved and CE pending. Synthetic MR’s SyMRI 3D is CE approved and 510(k) pending.
[3] Compared to Philips’ SENSE imaging.