Innovation spotlight: Advancing Cardiac Computed Tomography (CT) Imaging
Sep 26, 2025 | 4 minute read
Cardiac disease remains the biggest cause of death worldwide [1]. As a result, cardiac imaging plays an increasingly important role when it comes to diagnosing and treating these diseases.
Over the last 20 years, treatments have advanced tremendously but so has the imaging technology that works so critically alongside it. Here, Mani Vembar, Principal Scientist, CT Clinical Science, shares how Philips has been at the forefront of this important work and the added value that imaging now brings to cardiovascular care.
The invention of CT in the early 1970s ushered in a new era in medical imaging but imaging of moving organs still posed a significant challenge. The field of cardiology has been the primary driver of innovation in CT over the past 25 years, making a non-invasive approach to detect and rule-out heart disease possible.
The early years: from promise to practice
In the early days of cardiac CT, slow speeds, limited anatomical coverage, and radiation dose concerns were fundamental limitations to reliably achieving diagnostic-quality scans. While other organs are somewhat stationary, the heart is constantly in a dynamic state, pumping blood through small, rapidly moving coronary arteries.
Advancements in imaging technology over the past two decades have addressed these limitations, significantly improving cardiac CT performance. There are many cardiac diseases that can be detected by CT, but the guidelines are specific for coronary artery disease (CAD). For example, the American College of Cardiology (ACC) and European Society of Cardiology (ESC) recommend cardiac CT as the first-line test when it comes to ruling out CAD [2] [3].
The slice wars, the dose wars
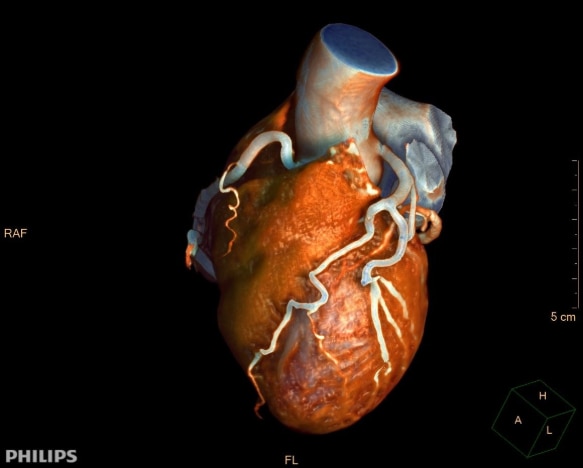
Anatomical coverage is important in cardiac CT and is commonly quantified as the number of ‘slices.’ Each increase in slice count translates directly into expanded coverage, reduced scan times, and improved success rates. In response, Philips progressed CT technology with its groundbreaking NanoPanel modular detector. This enabled rapid and continuous image quality improvements and increasingly larger coverage – and most importantly, expanding the population of patients who could benefit from cardiac CT.
However, dose challenges remained. With Step & Shoot Cardiac, Philips reduced radiation exposure by 80% [4] by using electrocardiograph (ECG) signals to limit x-ray exposure to a single moment within each heartbeat – unlike traditional gated helical scans, which expose patients to higher radiation levels by capturing images throughout the cardiac cycle.
Philips continued to further reduce radiation dose throughout the imaging chain: iDose4, an innovative reconstruction algorithm, improved image quality by increasing resolution, lowering noise and preventing artifacts [5], while knowledge-based iterative model reconstruction (IMR) pushed this even further to deliver clear images at extraordinarily low radiation doses [6].
A brilliant leap for cardiac care
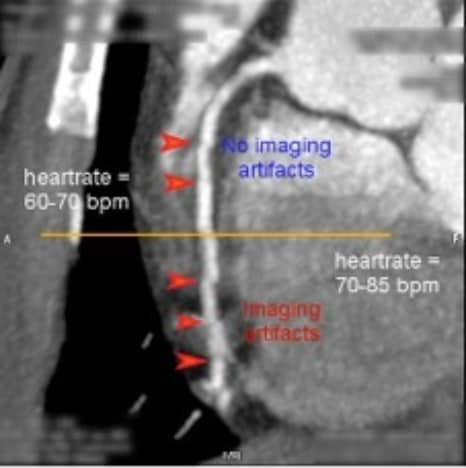
Philips continued to navigate the technical complexity of cardiac CT with sophisticated algorithms, including adaptive multi-cycle reconstruction that optimized temporal resolution and beat-to-beat variable delay algorithms that captured consistent cardiac phases, even as heart rates varied during scans [7] [8].
The introduction of Philips' Brilliance iCT marked a watershed moment in what cardiac CT could achieve. With a detector that could encompass the entire heart in just two acquisitions, the result enabled faster scans, higher success rates and significantly better image quality over a wider range of heart rates – with up to 80% dose reduction [9]. This was the entry point for cardiac CT as a mainstream clinical tool.
Spectral CT: the next frontier
By the mid-2010s, conventional cardiac CT challenges were largely solved. Philips then further advanced the field with detector-based spectral CT technology that simultaneously captured both high- and low-energy photon information in one scan – without additional dose, time, or workflow impact [10].
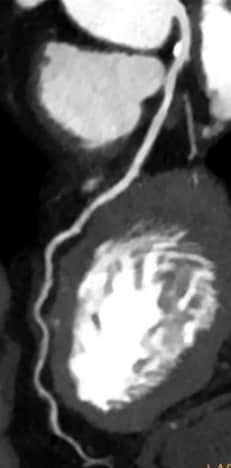
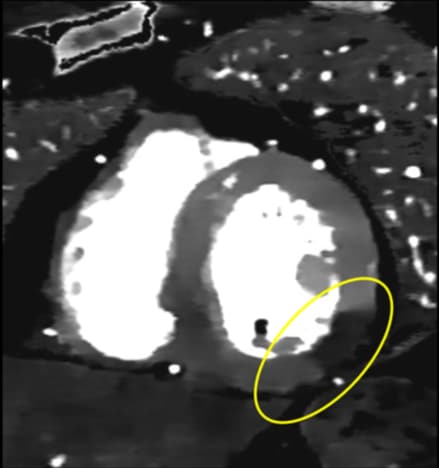
Spectral CT had a huge impact: reducing blooming in calcified plaques, optimizing visualization while reducing contrast agent doses, assessing myocardial perfusion [11], and improving evaluation around metallic coronary stents – truly providing a "one-stop shop" for comprehensive cardiac assessment [12] [13] [14].
These recent spectral CT innovations have helped meet the need for reliable non-invasive cardiac imaging that can complement existing care pathways. The ability to not only detect the presence of CAD but also its downstream impact makes CT a very powerful modality in facilitating improved risk assessment of patients.
Making cardiac CT smart
Incorporating the latest software solutions with hardware/technology is vital to meet the further increase in demand for advanced imaging in cardiac CT. Philips' most recent cardiac imaging innovations have been in the field of image reconstruction. Precise Cardiac compensates for heart motion with intelligent algorithms to reduce motion artifacts [15]. Precise Image employs AI to simultaneously reduce noise, improve image quality, and enable lower radiation dose [16]. Advancements like these enable routine high-quality cardiac scans to be performed swiftly and with lower radiation doses, for wider cohorts of patients.
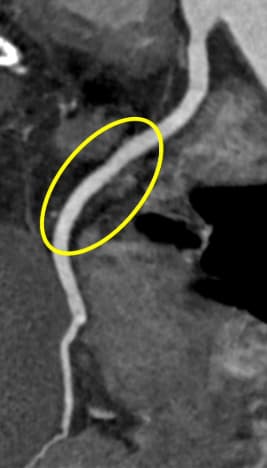
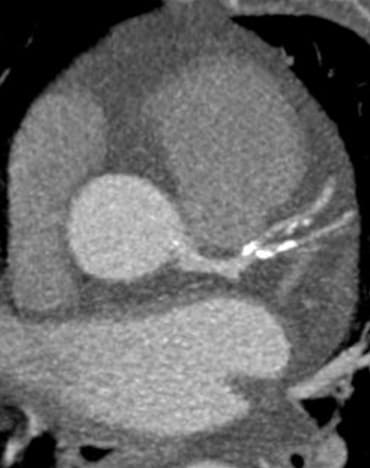
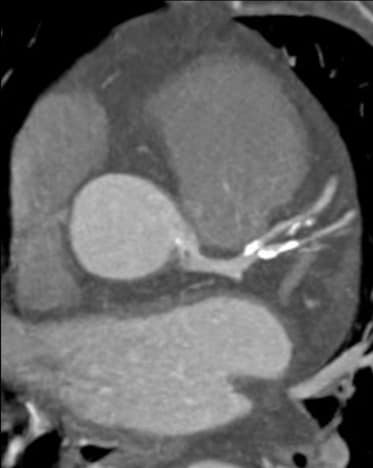
Leaps and bounds in cardiac innovation
The role of technologic advancements remains vital to cardiac CT and when it comes to innovation, at Philips we are just getting started. Stay tuned as we continue to accelerate the transformation through AI-powered solutions, to enable a future of better cardiac care for more people.
Sources [1] World Health Organization. Cardiovascular diseases. https://www.who.int/health-topics/cardiovascular-diseases (Accessed: September 2025). [2] Knuuti J, Wijns W, Saraste A, et al. 2019 ESC guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart J. 2019 Aug 31. PMID: 31504439. [3] Gulati M, Levy PD, Mukherjee D, et al. 2021 AHA/ACC/ASE/CHEST/SAEM/SCCT/SCMR Guideline for the Evaluation and Diagnosis of Chest Pain: A Report of the American College of Cardiology/American Heart. Association Joint Committee on Clinical Practice Guidelines. Nov 20: Circulation 144(22):e368-e454. DOI: 10.1161/CIR.0000000000001029. Epub 2021 Oct 28. PMID: 34709879. [4] Klass O, et al. Prospectively gated axial CT coronary angiography: preliminary experiences with a novel low-dose technique. Eur Radiol. 2009 Apr;19(4):829-36. doi: 10.1007/s00330-008-1222-4. Epub 2008 Nov 15. PMID: 19011864. [5] Hou Y, et al. Comparisons of image quality and radiation dose between iterative reconstruction and filtered back projection reconstruction algorithms in 256-MDCT coronary angiography. AJR 2012; 199(3):588–594. DOI: 10.2214/AJR.11.7557. PMID: 22915398. [6] Halliburton SS, et al. The role of advanced reconstruction algorithms in cardiac CT. Cardiovasc Diagn Ther. 2017;7(5):527–38. DOI: 10.21037/cdt.2017.08.12. PMID: 29255694. [7] Vembar M, et al. A dynamic approach to identifying desired physiological phases for cardiac imaging using multislice spiral CT. Med Phys. 2003 Jul;30(7):1683–93. DOI: 10.1118/1.1582812. PMID: 12906185. [8] Manzke R, et al. Adaptive temporal resolution optimization in helical cardiac cone beam CT reconstruction. Med Phys. 2003;30:3072–80. DOI: 10.1118/1.1624756. PMID: 14713073. [9] Diagnostic and Interventional Cardiology. Philips’ New 256-Slice CT Scans Heart in Two Beats. Diagnostic and Interventional Cardiology. 2007. Available at: https://www.dicardiology.com/content/philips-new-256-slice-ct-scans-heart-two-beats (Accessed: September 2025). [10] Rassouli N, et al. Detector-based spectral CT with a novel dual-layer technology: principles and applications. Insights Into Imaging. 2017;8(6):589–98. DOI: 10.1007/s13244-017-0571-4. PMID: 28986761. [11] Mochizuki J, et al. Spectral imaging with dual-layer spectral detector computed tomography for the detection of perfusion defects in acute coronary syndrome. Heart Vessels. 2022 Jul;37(7):1115-1124. doi: 10.1007/s00380-021-02019-2. Epub 2022 Jan 10. PMID: 35006370 [12] Xu C, et al. Incremental improvement of diagnostic performance of coronary CT angiography for the assessment of coronary stenosis in the presence of calcium using a dual-layer spectral detector CT: validation by invasive coronary angiography. Int J Cardiovasc Imaging. 2021;37(8):2561–72. DOI: 10.1007/s10554-021-02205-3. PMID: 34176031. [13] Cavallo AU, et al. Low dose contrast CT for transcatheter aortic valve replacement assessment: Results from the prospective SPECTACULAR study (spectral CT assessment prior to TAVR). J Cardiovasc Comput Tomogr. 2020;14(1):68–74. DOI: 10.1016/j.jcct.2019.06.015. PMID: 31416723. [14] Hickethier T, et al. Monoenergetic Reconstructions for imaging of Coronary Artery Stents Using Spectral Detector CT : In-vitro comparison to Conventional Images. J Cardiovasc Comput Tomogr. 2017;11(1):33–9. DOI: 10.1016/j.jcct.2016.12.005. PMID: 28096049. [15] Tetteroo PM, et al. Motion compensated reconstruction improves image quality and interpretability of dual-layer coronary CT angiography. Eur Radiol. 2025:10.1007/s00330-025-11946-x. Dobrolinska MM, et al. The influence of motion-compensated reconstruction on coronary artery analysis for a dual-layer detector CT system: a dynamic phantom study. Eur Radiol. 2024 Aug;34(8):4874-4882. doi: 10.1007/s00330-023-10544-z. Epub 2024 Jan 4. PMID: 38175219 [16] Barca P, et al. Image quality evaluation of the Precise image CT deep learning reconstruction algorithm compared to Filtered Back-projection and iDose4: a phantom study at different dose levels. Physica Medica. 2023;106:102517. DOI: 10.1016/j.ejmp.2022.102517.
Share this page with your network