Aug 25, 2020
Philips and B. Braun receive FDA clearance for breakthrough Onvision needle tip tracking technology for regional anesthesia
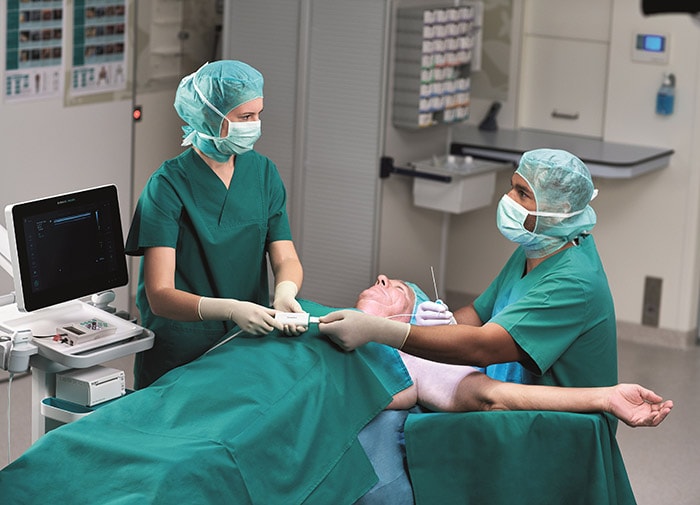
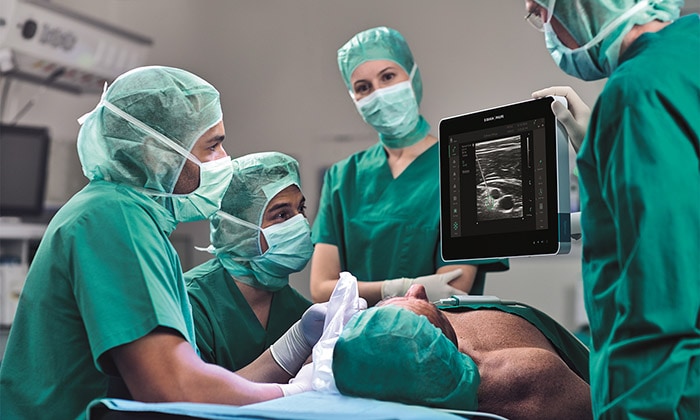
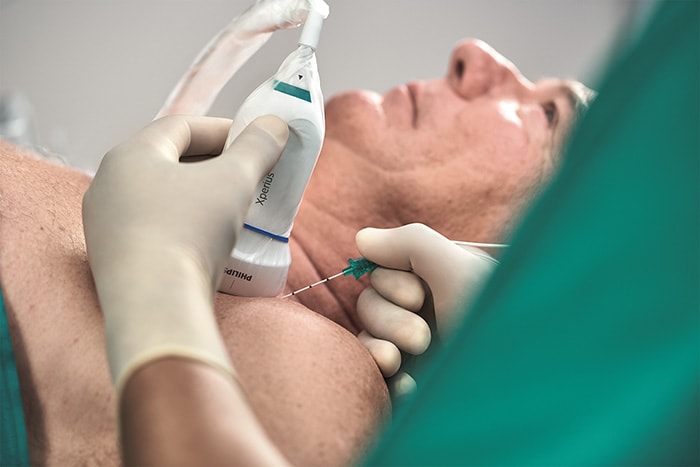
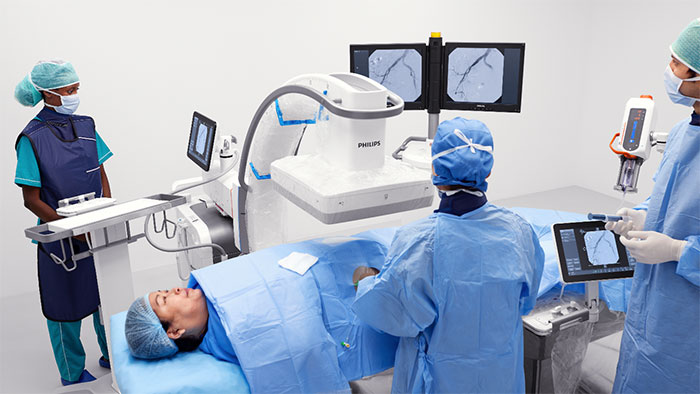
Amsterdam, the Netherlands – Royal Philips (NYSE: PHG, AEX: PHIA), a global leader in health technology, and B. Braun, a global market leader [1] in regional anesthesia and pain management, today announced 510(k) clearance from the U.S. Food and Drug Administration (FDA) for Onvision, a breakthrough ultrasound guidance solution for real-time needle tip tracking. Available exclusively on the latest version of the B. Braun and Philips Xperius ultrasound system together with the dedicated Stimuplex Onvision needle, Onvision gives anesthesiologists the confidence to accurately position the needle tip inside the body for Peripheral Nerve Blocks (PNBs). Accurate needle placement is critical to the success of regional anesthesia procedures such as PNBs. While real-time ultrasound imaging has proved to be a valuable tool for needle guidance, failure to optimally visualize the needle tip remains a challenge, with 10-15% of all PNBs ineffective after a single-injection technique [2]. In real-time, Onvision accurately indicates where the needle tip is inside the body, both in and out of the ultrasound viewing plane [3]. It helps the user align the needle with the probe in a user-friendly interface that can lead to a reduction in procedural time [4]. “When I first started to use Onvision, I didn’t think that we would increase the number of out-of-plane procedures, but I was happily surprised we did,” said Paul Kessler M.D. Ph.D., Vice Chairman, Clinic of Anesthesiology, Intensive Care Medicine and Pain Therapy, University Hospital Frankfurt (Germany). “Onvision gives you the extra assurance to perform deep and difficult blocks both in- and out-of-plane.” FDA clearance for Onvision represents the latest advance in a multi-year strategic alliance between Philips and B. Braun to innovate in ultrasound-guided regional anesthesia, a rapidly growing alternative to general anesthesia. Regional anesthesia is an essential part of pain therapy and offers clear advantages when compared to general anesthesia such as pain elimination during and after surgery [5], improved functional outcomes [6], and faster mobilization [7].
The increased confidence and predictability offered by the Xperius Ultrasound System and Onvision Needle Tip Tracking is empowering more anesthesiologists to embrace regional anesthesia as a viable and effective alternative to general anesthesia.
Tobin Taylor-Bhatia
Head of Innovation for Image Guided Therapy at Philips
“The increased confidence and predictability offered by the Xperius Ultrasound System and Onvision Needle Tip Tracking is empowering more anesthesiologists to embrace regional anesthesia as a viable and effective alternative to general anesthesia,” said Tobin Taylor-Bhatia, Head of Innovation for Image Guided Therapy at Philips. “By innovating together with B. Braun we’ve created a solution to one of the biggest challenges in regional anesthesia – accurate positioning of the needle tip in the body.” “Onvision is the groundbreaking technology that allows anesthesiologists the predictability [4] and confidence [4] they need while positioning the needle during a procedure,” said Dr. Angela Karpf, M.D., Corporate Vice President of Medical Affairs at B. Braun. “B. Braun continues to add products to our regional anesthesia therapy portfolio that help achieve faster postsurgical recoveries, optimize procedural workflow and enhance user experience.” Together, B. Braun’s Stimuplex Onvision needles and Philips’ Onvision needle tip tracking technology indicate the position of the needle tip in relation to the ultrasound viewing plane to an accuracy of 3mm [3]. A sensitive micro-sensor placed on the needle, combined with advanced signal processing and visualization techniques on the Xperius system, indicate the real-time location of the needle tip in relation to the 2D ultrasound viewing plane. The solution provides greater flexibility in needle trajectory [8] and can reduce procedure times [4]. In addition to FDA clearance, the Onvision solution is CE marked. It is available for sale across the EU and in Chile and is expected to be available in the United States in Q4 2020. For more information about Onvision visit bbraun.com/onvision. [1] 2019Q2 All Regional Anesthesia Products – Total Market HC & OPM Dollars (GHX, IQVIA [MDSA] & B. Braun Actual Data) [2] Paqueron X. Time Sequence of Sensory Changes after Upper Extremity Block. Anesthesiology 2004; 101:162-8 [3] Test Report Validation - NTT for PNB, Document Number: D000245353, Philips Medical Systems Nederland B.V. [4] Kåsine, T., et al. Needle tip tracking for ultrasound‐guided peripheral nerve block procedures—An observer blinded, randomised, controlled, crossover study on a phantom model. Acta Anaesthesiol Scand. 2019;00:1–8. https: //doi. org/10.1111/aas.13379 [5] Liu SS. Strodtbeck WM, Richman JM. Wu CL. A comparison of regional versus general anesthesia for ambulatory anesthesia: A meta-analysis of randomized control trials. Anesth Analg. 2005 Dec;101(6):1634-42 [6] Cohen NP, Levine WN, Marra G, et al. Indwelling interscalene catheter anesthesia in the surgical management of stiff shoulder: a report of 100 consecutive cases. J Shoulder Elbow Surgery. 2000;9:268-274 [7] Nielsen et al. Outcomes after RA. Int Anesthesiol Clin. 2005 Summer; 43(3):96 [8] Test Report Validation - NTT for PNB, Document Number: D000245352, Philips Medical Systems Nederland B.V. Onvision needle is manufactured by B Braun and the Onvision module for Xperius is manufactured by Philips. Onvision is a registered trademark of B. Braun and Philips. Stimuplex is a registered trademark of B. Braun Medical Inc.
About Royal Philips
Royal Philips (NYSE: PHG, AEX: PHIA) is a leading health technology company focused on improving people's health and enabling better outcomes across the health continuum from healthy living and prevention, to diagnosis, treatment and home care. Philips leverages advanced technology and deep clinical and consumer insights to deliver integrated solutions. Headquartered in the Netherlands, the company is a leader in diagnostic imaging, image-guided therapy, patient monitoring and health informatics, as well as in consumer health and home care. Philips generated 2019 sales of EUR 19.5 billion and employs approximately 81,000 employees with sales and services in more than 100 countries. News about Philips can be found at www.philips.com/newscenter.
About B. Braun
B. Braun Medical Inc., a leader in infusion therapy and pain management, develops, manufactures, and markets innovative medical products and services to the healthcare industry. Other key product areas include nutrition, pharmacy admixture and dialysis. The company is committed to eliminating preventable treatment errors and enhancing patient, clinician and environmental safety. B. Braun Medical is headquartered in Bethlehem, Pennsylvania and is part of the B. Braun Group of Companies in the U.S., which includes B. Braun Interventional Systems, Aesculap and CAPS. Globally, the B. Braun Group of Companies employs more than 64,000 employees in 64 countries. Guided by its Sharing Expertise philosophy, B. Braun continuously exchanges knowledge with customers, partners and clinicians to address the critical issues of improving care and lowering costs. To learn more about B. Braun Medical, explore our website.
Topics
Contacts

Kathy O'Reilly
Philips Global Press Office Tel.: +1 978-221-8919
You are about to visit a Philips global content page
ContinueAllison Longenhagen
B. Braun Medical Inc. Tel.: +1 484 523 9801
Media assets
Press releases
Get our press releases by e-mail
You are about to visit a Philips global content page
Continue