Apr 06, 2021
Philips SmartCT 3D image acquisition, visualization and measurement software for its Azurion Image Guided Therapy System receives FDA 510(k) clearance
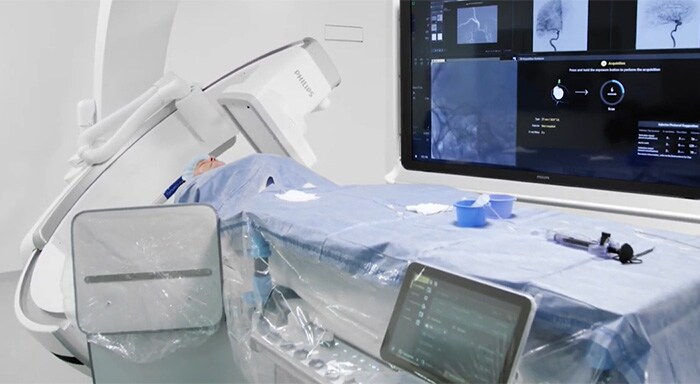
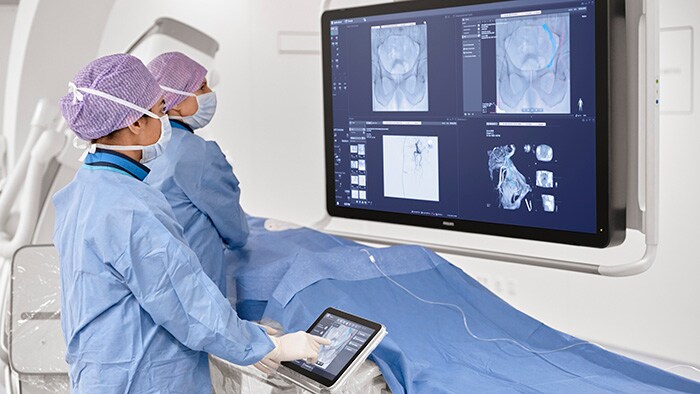
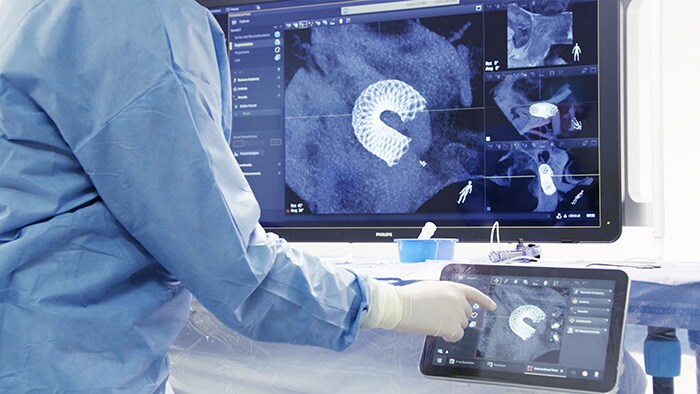
Amsterdam, the Netherlands – Royal Philips (NYSE: PHG, AEX: PHIA), a global leader in health technology, today announced U.S. Food and Drug Administration 510 (k) clearance for its Philips SmartCT application software. SmartCT is a key component of Philips Image Guided Therapy System – Azurion – providing interventionalists with CT-like 3D images (Cone Beam CT) to support diagnosis, therapy planning, treatment and follow-up for interventional radiology procedures. Bringing intuitive touchscreen control of advanced 3D image acquisition, visualization, vessel/organ segmentation, and quantitative measurements to the table-side, within the interventional lab’s sterile zone, SmartCT helps enhance clinical confidence, smooths workflows, and increases productivity. It includes software applications for angiography, neurology, soft-tissue imaging, and guidewire/catheter navigation, supporting a wide range of procedures such as the treatment of aneurysms, vascular diseases and liver tumors. Philips’ latest Azurion image-guided therapy platform integrates essential lab systems and tools needed for complex interventional procedures into an uncluttered laboratory environment in which interventionalists can focus on treating the patient rather than being distracted by the technology. SmartCT brings total control of the Azurion platform to a touchscreen tablet situated alongside the interventional radiology table. This eliminates the need for clinicians to leave the sterile field and step into an adjacent control room, as well as supporting faster and better-informed decision making.
A key part of our image-guided therapy strategy is to combine high-quality, low X-ray dose imaging with a superior user experience that allows interventional radiologists to diagnose and treat patients as part of smoother, safer and less interrupted workflows.
Ronald Tabaksblat
General Manager Image Guided Therapy Systems at Philips
“A key part of our image-guided therapy strategy is to combine high-quality, low X-ray dose imaging with a superior user experience that allows interventional radiologists to diagnose and treat patients as part of smoother, safer and less interrupted workflows,” said Ronald Tabaksblat, General Manager Image Guided Therapy Systems at Philips. “Philips SmartCT is a major step forward in 3D imaging, enhancing confidence in the interventional suite and supporting key elements of the quadruple aim of better patient outcomes, enhanced patient and staff experiences and lower cost of care.”
“Changing to a new technology can be challenging, but if the system itself can show you the way, it makes it much easier to adopt new advances,” Prof. Hicham Kobeiter, Chief of Radiology and Interventional Radiology, Hôpitaux Universitaires Henri-Mondor in Créteil, France. “SmartCT leads you through each step of the procedure, bringing us more confidence and more precision across cardiovascular, oncology and emergency cases.” “With the new SmartCT interface we can go into more detail more quickly and safely, with fewer staff in the room,” said Prof. Marc Sopval, Interventional Radiologist at Hôpital Européen Georges Pompidou AP-HP in Paris, France. “SmartCT has brought Cone Beam CT to life in everyday practice: all the tools and guidance capabilities are used by the entire team each day.” With SmartCT, users are guided through the image acquisition process and can review and interact with the acquired CT-like 3D images on the system’s table-side touch screen module using 3D visualization and measurement tools. These tools have been designed to support procedures in a range of clinical domains, including neurology, oncology, and cardiovascular procedures, and feature intuitive two-point distance measurements on 3D images, the ability to remove structures from the images that obstruct the region of interest, and the ability to select and store optimum projection angles for recall during procedures. Philips SmartCT image acquisition, visualization and measurement software is an integral part of the next-generation Philips Image Guided Therapy System – Azurion – which was launched in September 2020, marking an important step forward in optimizing clinical and operational lab performance and expanding the role of image-guided interventions in the treatment of patients. Azurion has achieved rapid global adoption, reflecting the accelerating trend toward minimally-invasive surgery thanks to its benefits in terms of reduced patient trauma, shorter recovery times and hospital stays, and lower healthcare costs. [1] Evaluated with clinical users in a simulated lab environment with a total of 17 teams consisting of a physician and an interventional radiology technologist with different levels of experience.
About Royal Philips
Royal Philips (NYSE: PHG, AEX: PHIA) is a leading health technology company focused on improving people's health and well-being, and enabling better outcomes across the health continuum – from healthy living and prevention, to diagnosis, treatment and home care. Philips leverages advanced technology and deep clinical and consumer insights to deliver integrated solutions. Headquartered in the Netherlands, the company is a leader in diagnostic imaging, image-guided therapy, patient monitoring and health informatics, as well as in consumer health and home care. Philips generated 2020 sales of EUR 17.3 billion and employs approximately 78,000 employees with sales and services in more than 100 countries. News about Philips can be found at www.philips.com/newscenter.
Topics
Contacts

Mark Groves
Philips Global Press Office Tel.: +31 631 639 916
You are about to visit a Philips global content page
Continue
Fabienne van der Feer
Philips Image Guided Therapy Tel: + 31 622 698 001
You are about to visit a Philips global content page
ContinueMedia assets
Press releases
Get our press releases by e-mail
You are about to visit a Philips global content page
Continue