Jul 07, 2021
Philips announces first patient enrolled in the WE-TRUST global multicenter stroke study to assess the impact of the Direct to Angio Suite workflow in improving patient outcomes
Amsterdam, the Netherlands – Royal Philips (NYSE: PHG, AEX: PHIA), a global leader in health technology, today announced that the first patient has been enrolled in the WE-TRUST study at Vall d’Hebron University Hospital, Barcelona, Spain, marking the official start of this major multicenter randomized controlled trial to assess if the Direct to Angio Suite workflow can improve outcomes for early time-window stroke patients (less than six hours after stroke onset).
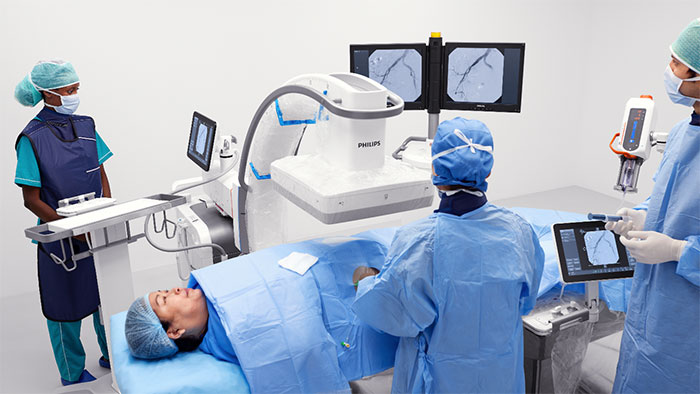
The WE-TRUST (Workflow optimization to rEduce Time to endovascular Reperfusion for Ultra-fast Stroke Treatment) trial will study the clinical impact of the Direct to Angio Suite approach, enabled by a special scan in the angio suite developed by Philips. This approach combines stroke diagnosis and subsequent treatment in the same angio suite, which the study will compare to the conventional workflow of diagnosing patients for treatment in the CT or MRI suite and then treating them in a separate angio suite. The Direct to Angio Suite approach can potentially reduce the time to treatment for early time-window stroke patients for whom every second counts. WE-TRUST will be the first global multicenter randomized controlled trial to assess the impact of the Direct to Angio Suite approach. Besides this clinical trial for early time-window stroke patients, Philips is committed to optimizing the care pathway for stroke patients in general.
“After suffering a stroke, fast time to treatment is paramount to giving patients the best possible outcomes. What we will learn about improving time to treatment in the WE-TRUST trial has the potential to significantly improve how acute stroke patients are diagnosed and treated,” said Dr. Raul Nogueira, Director, Neuroendovascular Service Marcus Stroke and Neuroscience Center at Grady Health in Atlanta, Georgia, and WE-TRUST Principal Investigator. “With the help of an advanced brain scan technology in the angio suite, for example, we intend to eliminate the need for conventional CT or MRI scans for stroke triage in select patients to save valuable time.”
This multicenter, prospective, randomized, controlled, open-label, blinded-endpoint trial is intended to take place at 15 leading strokes sites and aims to enroll more than 560 patients across the United States, Brazil, Argentina, the Netherlands, France, Germany, Spain and Turkey. The trial is anticipated to be completed by 2023.
Stroke is the leading cause of disability
Globally, 1 in 4 adults over the age of 25 will have a stroke in their lifetime [1], and data show that 1 in 200 people are stroke survivors, making acute stroke the second leading cause of death and a leading cause of serious long-term disability [2]. Currently, when a possible stroke patient arrives at the emergency department, they typically first undergo a CT or MRI exam and, in the case of an ischemic stroke, are then treated in an interventional suite. Outcomes for stroke patients are closely tied to how quickly they receive treatment: every 30 minutes’ delay before treatment reduces the chance of a good outcome by 14% [3], and every hour of delay ages the brain by 3.6 years compared to a normally aging brain [4]. Various single-center studies have already shown that a Direct to Angio Suite workflow can result in time-savings up to 54 minutes with improved patient outcomes after 90 days [5].
Advanced brain scan in the angio suite
The Direct to Angio Suite workflow is enabled by an advanced brain scan in the angio suite, developed by Philips, that uses improved cone-beam computed tomography (CBCT) to improve image quality and facilitate triage of patients. This investigational device reconstructs the stroke CBCT images using specially designed algorithms and filters to reduce artifacts caused by patient motion, bone beam hardening and metal objects.
“In a single center randomized clinical trial in our center, the Direct to Angio Suite workflow has shown a significant improvement in clinical outcomes in patients who suffered a stroke,” said Dr. Marc Ribó, WE-TRUST co-Principal Investigator, Interventional Neurologist at the Vall d’Hebron University Hospital and researcher at the Stroke Research group at the Vall d’Hebron Research Institute (VHIR). “With the updated Stroke CBCT investigational device we can better facilitate triage of patients through improved image quality.”
The WE-TRUST study is a key milestone, building on Philips’ leading position in image guided therapy and our strong global network of clinical partnerships. It adds to our deep commitment to further optimize stroke workflow, remove the hurdles to fast, decisive treatment, and improve stroke outcomes.
Dr. Atul Gupta
Chief Medical Officer for Image Guided Therapy at Philips
“We are very excited to initiate this multicenter study, partnering with leading stroke centers and physicians to innovate the diagnosis and treatment of stroke patients,” said Dr. Atul Gupta, Chief Medical Officer for Image Guided Therapy at Philips. “The WE-TRUST study is a key milestone, building on Philips’ leading position in image guided therapy and our strong global network of clinical partnerships. It adds to our deep commitment to further optimize stroke workflow, remove the hurdles to fast, decisive treatment, and improve stroke outcomes.”
The trial will primarily be carried out on Philips Image Guided Therapy System – Azurion – the company’s leading system for interventional procedures. The primary endpoint of the WE-TRUST trial is clinical outcome measured by the patients’ functional status (mRS) three months after the procedure. More information about the study can be found at wetrust-study.com.
[1] https://www.world-stroke.org/world-stroke-day-campaign/why-stroke-matters/learn-about-stroke
[2] Centers for Disease Control and prevention (https://www.cdc.gov/stroke/facts.htm); Virani SS, Alonso A, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, et al. Heart disease and stroke statistics—2020 update: a report from the American Heart Associationexternal icon. Circulation. 2020;141(9):e139–e596. (https://www.ahajournals.org/doi/10.1161/CIR.0000000000000757)
[3] Mazighi, M. et al. Impact of Onset-to-Reperfusion Time on Stroke Mortality. Circulation 127, 1980-1985, doi:10.1161/circulationaha.112.000311 (2013). (https://www.ahajournals.org/doi/10.1161/circulationaha.112.000311)
[4] Saver, J. L. (2006). Time is brain—quantified. Stroke, 37(1), 263-266. (https://www.ncbi.nlm.nih.gov/pubmed/16339467)
[5] Mendez, B., et al., Direct Transfer to Angio-Suite to Reduce Workflow Times and Increase Favorable Clinical Outcome. Stroke, 2018. 49(11): p. 2723-2727. (https://www.ahajournals.org/doi/10.1161/STROKEAHA.118.021989)
About Royal Philips
Royal Philips (NYSE: PHG, AEX: PHIA) is a leading health technology company focused on improving people's health and well-being, and enabling better outcomes across the health continuum – from healthy living and prevention, to diagnosis, treatment and home care. Philips leverages advanced technology and deep clinical and consumer insights to deliver integrated solutions. Headquartered in the Netherlands, the company is a leader in diagnostic imaging, image-guided therapy, patient monitoring and health informatics, as well as in consumer health and home care. Philips generated 2020 sales of EUR 17.3 billion and employs approximately 78,000 employees with sales and services in more than 100 countries. News about Philips can be found at www.philips.com/newscenter.
Topics
Contacts

Joost Maltha
Philips Global Press Office Tel: +31 6 10 55 8116
You are about to visit a Philips global content page
Continue
Fabienne van der Feer
Philips Image Guided Therapy Tel: + 31 622 698 001
You are about to visit a Philips global content page
ContinueMedia assets
Vall d'Hebron University Hospital Spain
Clinical Team Vall d'Hebron
WE-TRUST logo
Press releases
Get our press releases by e-mail
You are about to visit a Philips global content page
Continue