Nov 23, 2021
Philips launches new AI-enabled MR portfolio of smart diagnostic systems, optimized workflow solutions and integrated clinical solutions at RSNA 2021
Amsterdam, the Netherlands – Royal Philips (NYSE: PHG, AEX: PHIA), a global leader in health technology, today announced new AI-enabled innovations in MR imaging launching at the Radiological Society of North America (RSNA) annual meeting (November 23 – December 2, Chicago, USA). Philips’ new MR portfolio of intelligent integrated solutions is designed to speed up MR exams, streamline workflows, optimize diagnostic quality, and help ensure the efficiency and sustainability of radiology operations. As MR imaging plays an ever-increasing role in precision diagnosis, radiology departments face multiple challenges such as workload demands due to the backlog of cases resulting from the COVID-19 pandemic, a growing population of clinically challenging patients, and a worldwide shortage of skilled technicians. As a result, there is a clear need for smart diagnostic systems, optimized workflows, and integrated clinical solutions that reduce time-to-diagnosis, improve patient throughput, reduce the need for rescans, and enhance the patient and staff experience.
At this year’s RSNA, we are focused on introducing scalable high performance MR systems to the imaging enterprise, with intelligent software built in to automate tasks to help relieve the burden on radiology staff and departments.
Arjen Radder
General Manager of MR at Philips
“At this year’s RSNA, we are focused on introducing scalable high performance MR systems to the imaging enterprise, with intelligent software built in to automate tasks to help relieve the burden on radiology staff and departments,” said Arjen Radder, General Manager of MR at Philips. “With AI-driven smart connected systems, optimized workflows, and integrated clinical solutions, our goal is to deliver high quality diagnostic images while also improving radiology department productivity by relieving radiologists of burdensome routine tasks so they have more time to take care of their patients.” Among several new AI-enhanced MR innovations being launched by Philips at RSNA 2021 are Philips SmartSpeed [3], designed to speed up image acquisition and enhance image quality and diagnostic confidence for every patient, and the company’s new MR Workspace with AI assistance, which delivers an intuitive solution to simplify the path from image acquisition to diagnosis to help improve MR workflow and staff experience. Other introductions include the company’s new MR 5300 and MR 7700 smart connected systems.
Sustainable and connected smart diagnostic systems
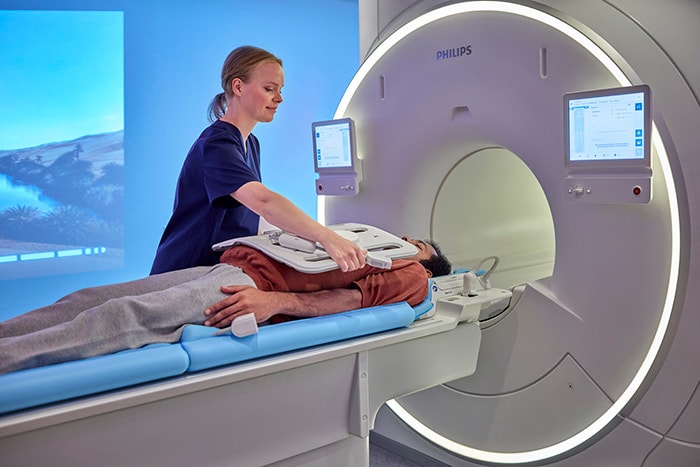
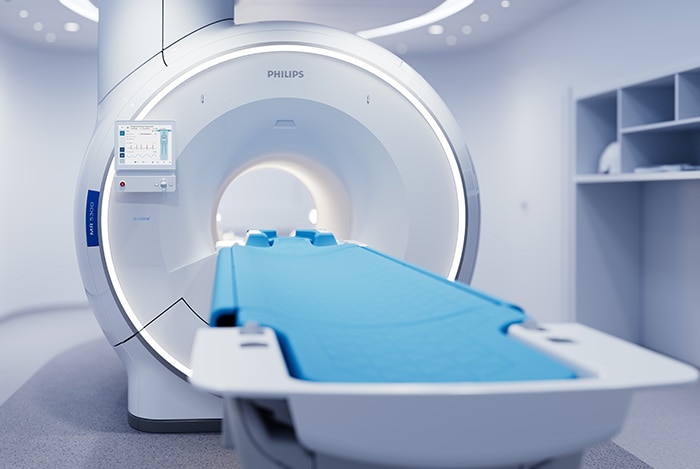
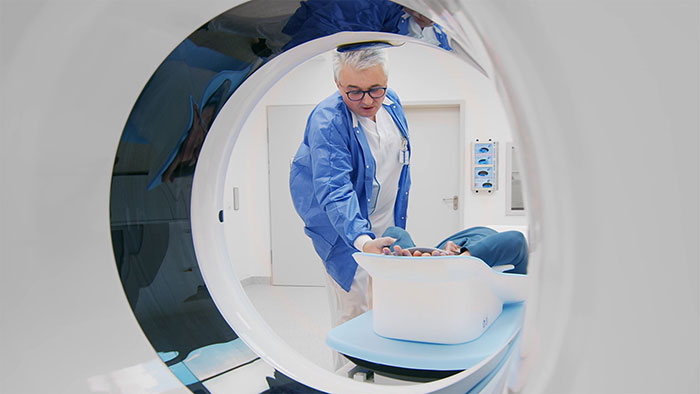
Philips’ new MR 5300 system being introduced for the first time at RSNA 2021, has received 510(k) clearance from the U.S. Food and Drug Administration (FDA). With this introduction, the company’s latest BlueSeal fully-sealed helium-free in operations MR scanner continues the advancement of a full helium-free operating portfolio of Philips MR solutions. Powered by AI, the MR 5300 simplifies and automates complex clinical and operational tasks for outpatient clinical use and MR departments to help increase access to affordable, quality care. “At RSNA, we continue our commitment to helium-free sustainability in MR operations with new and enhanced technology to provide fast, fully automated, and highly personalized exams for every patient without compromising image quality, while also acting responsibly towards the planet and society,” Radder added. The MR 5300’s large (55cm) field-of-view provides extended anatomical coverage, while its robust AI-powered scanning methods and easily positioned ultra-lightweight dStream Breeze coils [5] work together to generate exceptional image quality, especially when imaging challenging anatomies such as the spine and abdomen. By simplifying and automating many of the complex clinical and operational tasks involved in capturing high-quality images, MR 5300 enhances the consistency and productivity of radiology department output, helping to increase patient satisfaction scores and referral rates. Its helium-free operations make it suitable for use in outpatient clinics, as well as dedicated radiology departments.
Fully automated optimized workflows engine drives quality and efficiency
Also debuting at RSNA 2021, Philips’ new MR Workspace with AI assistance is designed to simplify the path from image acquisition to diagnosis and empower technologists – regardless of their experience or patient challenges – to drive productivity and predictability. MR Workspace’s intuitive dashboard fully automates the planning and execution of many routine scans and eliminates guesswork by suggesting the most suitable Exam Card for each patient to ensure consistency in radiology department operations. “MR Workspace provides us with a faster turnover time in between planned patients, allowing us to prepare the examination of the next patient while the current patient is still being scanned,” said Ronald Peeters, MR Physicist at University Hospital Leuven in Belgium. “With MR Workspace, our radiology department has been able to increase our scheduling efficiency and improve productivity [5].”
Ultrafast and personalized integrated clinical solutions
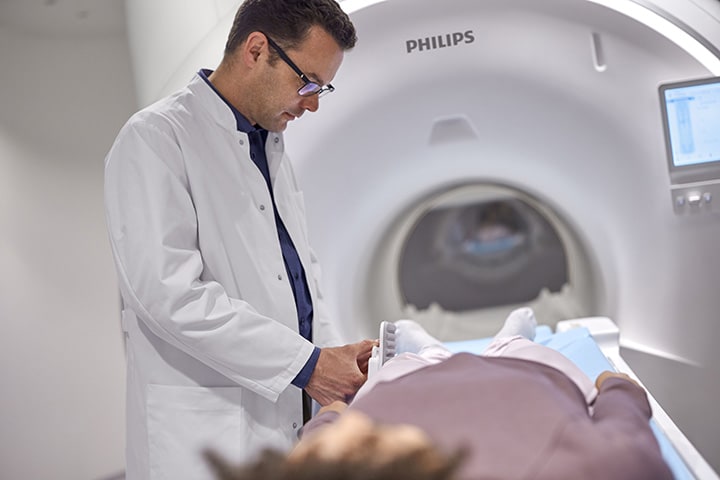
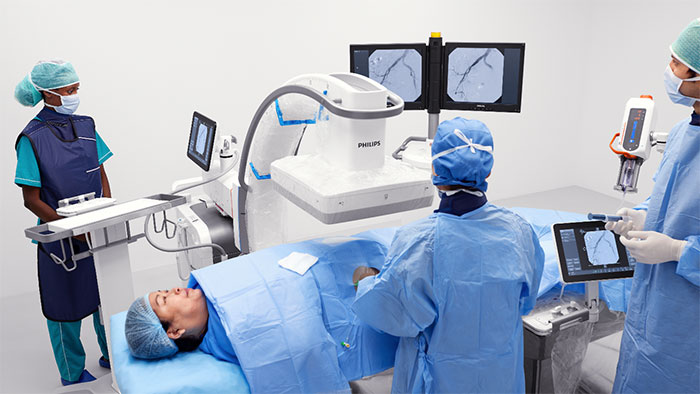
Another major reveal at RSNA 2021 is Philips’ new MR 7700 system [2] integrated with multinuclear clinical capabilities to give clinical researchers the confidence to explore new imaging pathways. Researchers can also leverage the accuracy, power, and endurance of the MR 7700’s XP gradients to help facilitate demanding research programs to boost neuroscience excellence, and leverage the built-in AI technology for very high-performance clinical practice. The MR 7700’s gradient is designed to deliver the highest level of performance, state-of-the-art diffusion imaging, excellent signal-to-noise ratio (SNR), and motion detection technology to support outstanding imaging results. The MR 7700 system’s high homogeneity and linearity bring precision to both anatomical and functional imaging, supporting confident decision making. The system aims to tackle existing, new, and future clinical and research demands, while addressing the upswing in patient volumes experienced by many of today’s radiology departments. With this latest release, Philips has expanded to five additional nuclei to advance clinical insight with more metabolic and functional information across anatomies and making Multi Nuclei imaging a part of the daily MR clinical workflow.
AI-powered technology driving high quality imaging for every patient
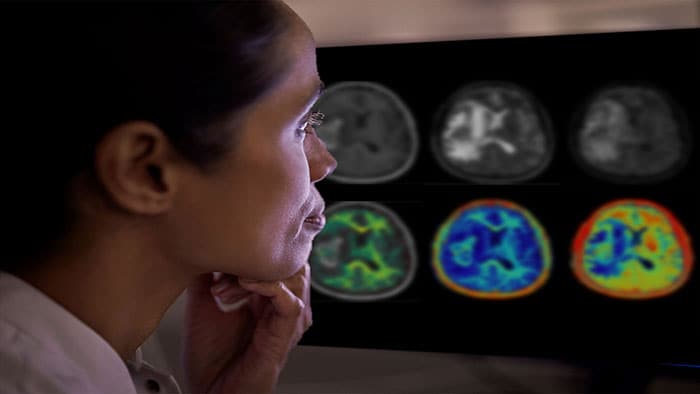
At RSNA 2021, Philips is also launching its new SmartSpeed [3] reconstruction platform, designed to support the latest AI innovations. The platform, which features an award winning AI algorithm (Adaptive-CS-Net) applied directly at the source of the MR signal, is the next big advance on Philips’ existing Compressed SENSE acceleration technology, further enhancing the ability to reconstruct a full image from under-sampled data while maintaining virtually equivalent image quality. The projected speed advantages will help radiology departments to significantly increase patient throughput and enhance diagnostic confidence in a wider range of patients, including patients who are in pain, who struggle to hold their breath during the exam, or who have MR conditional implants. The SmartSpeed platform is designed to support 97% of current clinical protocols. Join Philips at RSNA 2021 where the company will spotlight its advanced technology, including its latest AI-enhanced MR portfolio, driving connected workflows and smart connected imaging systems, to increase efficiency and diagnostic confidence in precision care and treatment. And follow @PhilipsLiveFrom for updates throughout the RSNA 2021 event. [1] According to the definition of AI from the EU High-Level Expert Group.
[2] Pending 510(k), Not available for sale in the U.S.A.
[3] Pending 510(k), Not available for sale in the U.S.A.
[4] Adaptive-CS-Net: FastMRI with Adaptive Intelligence. 33rd Conference on Neural Information Processing Systems (NeurIPS). Vancouver, Canada. Dec. 2019.
[5] Customer case study. Results may vary.
About Royal Philips
Royal Philips (NYSE: PHG, AEX: PHIA) is a leading health technology company focused on improving people's health and well-being, and enabling better outcomes across the health continuum – from healthy living and prevention, to diagnosis, treatment and home care. Philips leverages advanced technology and deep clinical and consumer insights to deliver integrated solutions. Headquartered in the Netherlands, the company is a leader in diagnostic imaging, image-guided therapy, patient monitoring and health informatics, as well as in consumer health and home care. Philips generated 2020 sales of EUR 17.3 billion and employs approximately 78,000 employees with sales and services in more than 100 countries. News about Philips can be found at www.philips.com/newscenter.
Topics
Contacts

Kathy O'Reilly
Philips Global Press Office Tel.: +1 978-221-8919
You are about to visit a Philips global content page
ContinueMedia assets
Press releases
Get our press releases by e-mail
You are about to visit a Philips global content page
Continue