Prostate cancer is the second most common cancer among men worldwide, after lung cancer [1]. Unfortunately, diagnosis and therapy selection are not always simple or straightforward. Many prostate cancers are indolent and unlikely to pose a life threat. This means that for a large proportion of patients, active surveillance may be a better option compared to active treatment, which can have debilitating side effects such as impotence or incontinence. Yet it is crucial to discriminate indolent prostate cancers from the more aggressive ones. Once prostate cancer has spread to distant parts of the body, survival rates drop to 30% [2]. This poses a difficult dilemma: should you avoid any risk of cancer progression and start treating immediately, at the expense of serious side effects? Or should you rather “watch and wait”, allowing the patient to enjoy a better quality of life as long as active treatment is not needed? There is a considerable risk of either overtreating or undertreating the patient.
With prostate cancer, there is a considerable risk of either overtreating or undertreating the patient.
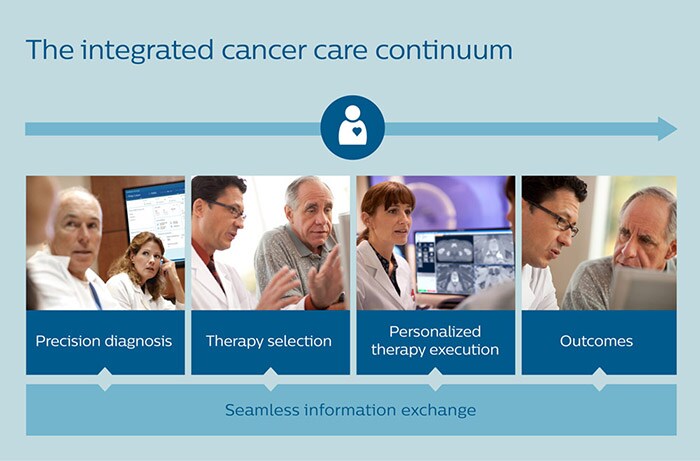
If active treatment is pursued, challenges compound. How do you create an optimal treatment plan for each individual patient? And, as more and more medical specialists get involved along the way, how do you jointly keep track of all relevant information about the patient and his disease? In my previous article, I outlined how an integrated, personalized approach to lung cancer care – all the way from precision diagnosis to treatment selection – can benefit patients and physicians alike. In this follow-up article, I will show how prostate cancer can be addressed in a similar way – taking the same approach even further by enabling personalized prognosis, therapy execution and monitoring.
Improving detection of significant prostate cancers
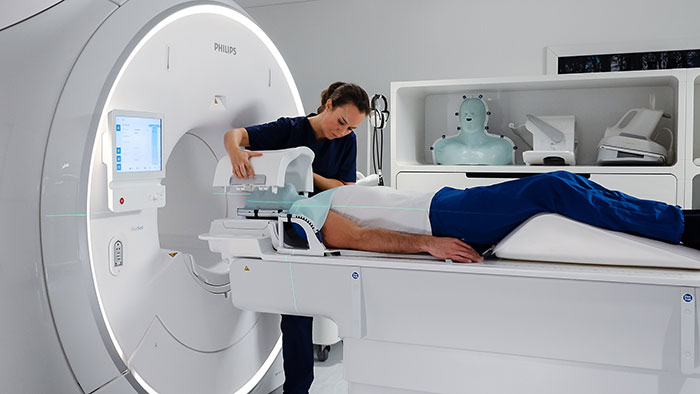
As a first step, we need more accurate methods for diagnosing prostate cancer and predicting how aggressively it will evolve. The traditional golden standard for diagnosing prostate cancer is transrectal ultrasound (TRUS) guided biopsy, in which a small tissue sample is taken from the prostate by inserting a thin, hollow needle via the rectum. During the procedure, the urologist will typically collect 12 to 14 tissue samples. The problem with this method is that it only samples up to 1% of the prostate, resulting in a risk of 30% of missing the cancer [3]. Over the past years, multi-parametric Magnetic Resonance Imaging (mpMRI) has gained prominence as a way of obtaining a more targeted and accurate biopsy. Based on MR images of the prostate, the radiologist can delineate suspicious lesions. Fusing these MR images with TRUS images in real time allows the urologist to see suspicious lesions while visualizing the biopsy needle at the same time – offering more precise guidance for biopsy-taking. Targeted biopsy-taking has decreased the average number of necessary biopsies from 12 (in traditional biopsy-taking) to 3 or 4, decreasing discomfort for the patient as well as risk of infection. Moreover, targeted biopsy-taking can improve detection of high-risk cancers by 30% compared to traditional biopsy-taking, while detecting 17% fewer low-risk cancers [4].
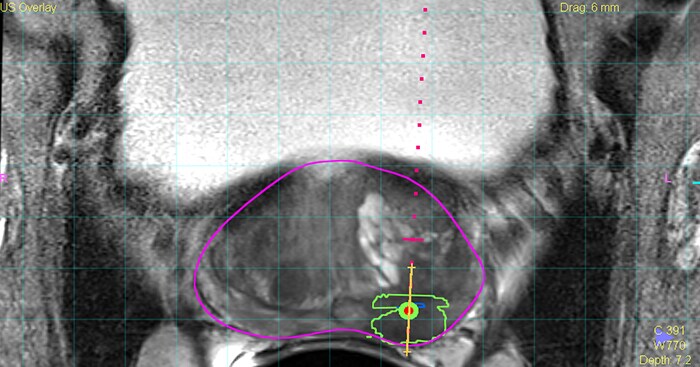
Fusing MR images of suspicious lesions in the prostate with live ultrasound images allows urologists to take more targeted and guided biopsies for diagnosis of prostate cancer. (Purple line = prostate; green line = suspicious lesion; yellow line = position of the biopsy needle.)
Taking the variability out of prognosis
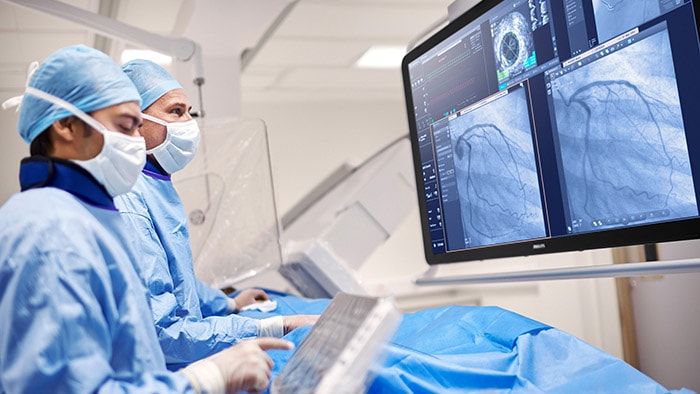
Through the use of MR images, strides have also been made in assessing the risk of prostate cancer with a scoring method known as Prostate Imaging Reporting and Data System (PI-RADS). However, assigning a score to a tumor based on a subjective interpretation of tumor features – such as size and shape – leaves room for variability from one observer to the other [5]. This is where I see huge potential for AI to support radiologists and urologists. And indeed, efforts are already underway to make PI-RADS scoring more objective using machine learning algorithms trained on large image sets. In a similar vein, pathologists who use digital pathology to inspect tissue samples obtained via a biopsy stand to gain from algorithms that aid them in their analysis, pointing them to areas of interest that demand closer inspection. Enabled by digital pathology, such algorithms can be trained on large numbers of high-resolution images of tissue samples to detect subtle patterns that may escape the human eye.
AI is poised to make risk assessment in patients with prostate cancer more objective.
Genomics and molecular diagnostics are also becoming an increasingly important part of precision diagnosis of prostate cancer. Tumor-specific gene expression can provide additional insights on how the cancer is likely to progress in an individual. For instance, prostate-specific antigen (PSA) testing – which has traditionally been used as a first blood-based screening method but leaves much to be desired in terms of reliability – is now being complemented with more specific blood- or urine-based molecular diagnostic methods (liquid biopsy tests) to identify patients at risk for significant prostate cancer.
Connecting the dots across disciplines
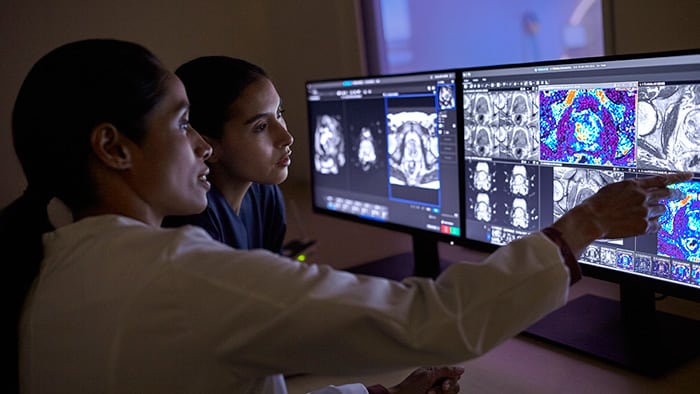
All this information will need to come together in a unified and comprehensive diagnosis and prognosis. This is where physicians often struggle today. Collection and reporting of patient data is fragmented, while the amount and complexity of available data is growing. Yet multidisciplinary teams typically only have a few minutes to discuss a patient during a tumor board meeting. Errors are prone to occur. As detailed in my previous article, we developed Philips IntelliSpace Precision Medicine Oncology to address this challenge – bringing together relevant information in shared dashboards and intelligent timelines to support cross-disciplinary collaboration, for better and more efficient decision-making. As the number of men with prostate cancer is expected to increase as populations age, quick and easy information sharing will be pivotal for efficiently handling mounting workloads while supporting precision diagnosis for each patient.

With Philips IntelliSpace Precision Medicine Oncology, different specialists have access to the same unified patient overview.
Making treatment selection more evidence-based
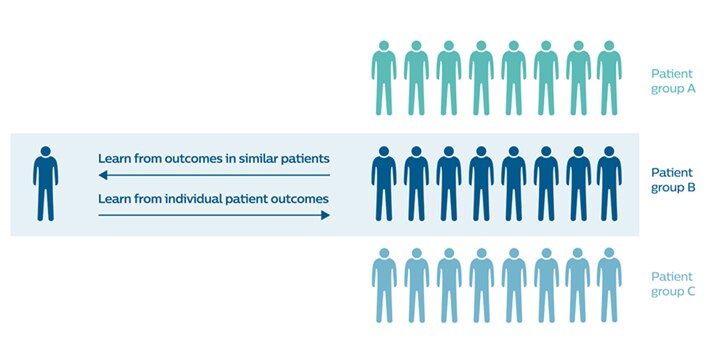
Once an initial diagnosis is made, selecting the appropriate treatment strategy presents new challenges. Should you pursue active treatment, typically in the form of surgery or radiation therapy, and if so, how intense should the treatment be? Or is active surveillance a more suitable strategy? The patient and the responsible physician are faced with a difficult trade-off: active and more intense treatment may improve the chances of survival without disease progression, but it can adversely affect quality of life because of possible complications and side effects such as urinary or sexual dysfunction. Insights into the aggressiveness of the cancer can help to make better informed decisions. But ideally, you would also like to have information on how certain treatment choices actually played out in similar patients. That is, if a therapy decision is needed for a 59-year old man with tumor characteristics A, B, and C, both patient and physician would benefit from insight into how other 59-year old men with tumor characteristics A, B, and C fared depending on a given treatment choice.
By analyzing large data sets with patient outcomes, both in terms of survival rates and quality of life, we can make therapy selection more personalized and evidence-based.
To make this type of information available, individual patient outcomes should feed into a larger body of outcome-based learnings (in an anonymized way). These learnings can then inform therapy selection for similar patients on an individual level. For example, as part of BigMedilytics – an EU-supported big data consortium led by Philips Research – we are working together with Karolinska University Hospital in Sweden to develop predictive modelling for prostate cancer surgery outcomes, which can help guide patients and physicians in their decisions. With the advance of AI, I believe that this kind of data-informed approach will become much more common. By analyzing large data sets with patient outcomes, both in terms of survival rates and quality of life, we can make therapy selection more personalized and evidence-based.
Supporting personalized radiation therapy
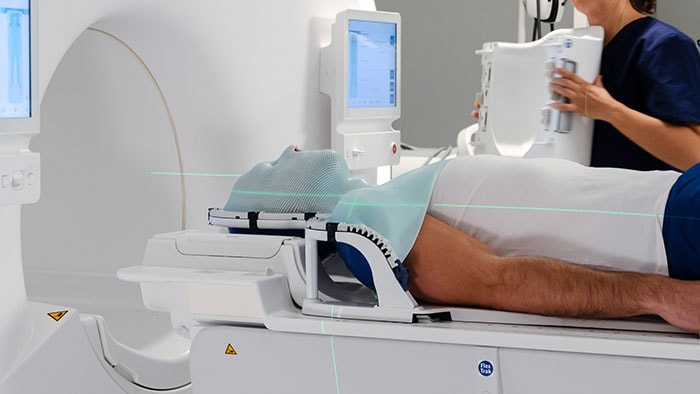
Personalizing prostate cancer care doesn’t stop at treatment selection. The execution of the treatment also involves a range of decisions that determine the effect of treatment on an individual. Probably no other treatment exemplifies this better than radiation therapy – which is the treatment of choice for 58% of men with prostate cancer [6]. In the most common type of radiation therapy, beams of radiation are focused on the tumor from a machine outside the body. This requires careful planning, to ensure that the tumor is exposed to the appropriate amount of radiation while minimizing radiation dose to healthy organs such as the bladder or the rectum. Managing this planning process can be incredibly challenging and time-consuming. The development of a radiation therapy plan relies on close collaboration between specialists from multiple disciplines. A radiation therapy workflow typically consists of many steps from the moment of patient referral to the start of treatment – including simulation imaging, mapping the contours of the tumor and surrounding structures, creating the treatment plan, and ensuring quality control. Each of these steps involves multiple checks and approvals by different stakeholders, with many – often manual – handovers. To address this complexity, we are developing a digital platform that is designed to integrate – and to a large extent automate – all different steps in the workflow of a radiotherapy department. The aim is to give specialists access to the same information at every point in time – enabling precise and personalized radiation therapy for each patient.
To support radiation therapy workflows, we are bringing together all relevant information in one place. Digital information sharing also makes it easier to set up a hub-and-spoke model for radiation therapy. Treatment plans can be prepared in one central hospital while the actual radiation therapy is delivered via local hubs. This saves vulnerable patients time and hassle, as they don’t have to travel as far for what is typically a series of 30 radiation therapy sessions spread over the course of six or more weeks.
Life after prostate cancer
Once the prostate cancer has been treated successfully, monitoring for recurrence is vital for long-term health outcomes. Up to 30% of men who received localized prostate cancer treatment will experience cancer recurrence – either in the tissue next to the prostate, in the surrounding lymph nodes, or in other parts of the body such as the bones [7]. This is where screening methods such as blood-based PSA testing (and more advanced forms of liquid biopsy) come into the picture again, followed up by additional imaging studies to localize suspicious lesions. An imaging technique known as Prostate-Specific Membrane Antigen (PSMA)-PET has shown to be particularly promising for identifying cancer recurrence, spotting tiny lesions that previously went undetected [8].
Once the prostate cancer has been treated successfully, monitoring for recurrence is vital for long-term health outcomes. This is where I foresee a growing role for home monitoring.
I also foresee a growing role for home monitoring, enabling patients to share outcomes – such as urinary and erectile function – with the hospital. Today, patients often have regular face-to-face consultations that require them to visit the hospital, even though they may be free of side effects or complications. In those cases, a consultation by phone may suffice, saving the patient a trip to the hospital while keeping them engaged. At the same time, monitoring patient-reported outcomes could support in identifying patients who are at risk of recurrence or who are experiencing side effects or complications – allowing healthcare professionals to intervene quickly when needed. It would be a fitting next step in personalizing cancer care: giving every patient the care they need, while allowing them to enjoy the best possible quality of life, close to their loved ones, for as long as possible. References [1] World Cancer Research Fund. https://www.wcrf.org/dietandcancer/cancer-trends/worldwide-cancer-data [2] National Cancer Institute. https://seer.cancer.gov/statfacts/html/prost.html [3] Health Europa. https://www.healtheuropa.eu/genomic-testing-prostate-cancer/90457/ [4] Siddiqui MM, Rais-Bahrami S, Turkbey B, et al. Comparison of MR/Ultrasound Fusion–Guided Biopsy With Ultrasound-Guided Biopsy for the Diagnosis of Prostate Cancer. JAMA. 2015;313(4):390-397. [5] Gupta, RT, & Rosenkrantz, AB. Prostate MRI can be accurate but can variability be reduced? Nature Reviews Urology. 2018;15:339-340. https://www.nature.com/articles/s41585-018-0002-4 [6] Chamie K, Williams SB, Hu JC. Population-Based Assessment of Determining Treatments for Prostate Cancer. JAMA Oncol. 2015;1(1):60–67. [7] Kolodziej M, Management of Biochemically Recurrent Prostate Cancer Following Local Therapy, Am. J. Manag. Care 2014;20: S273-281 https://www.ajmc.com/journals/supplement/2014/ace021_dec14_prostate_ce/ace021_dec14_prostatece_kolodziej [8] Barbosa FG, Queiroz MA, Nunes RF, Viana PCC, Marin JFG, Cerri GG, Buchpiguel CA, Revisiting Prostate Cancer Recurrence with PSMA PET: Atlas of Typical and Atypical Patterns of Spread, Radiographics. 2019;39(1):186-212. doi: 10.1148/rg.2019180079. https://www.ncbi.nlm.nih.gov/pubmed/30620699
Share on social media
Topics
Author

Henk van Houten
Former Chief Technology Officer at Royal Philips from 2016 to 2022