Tracing back to the days of Hippocrates around 400 B.C., cancer used to be spoken of as a single disease. It is an image that still lingers in the public perception of cancer. But in medicine today, we know that cancer really has multiple subtypes on a molecular level. Even cancers with the same site of origin, like lung cancer, can be very different from one person to the other – and respond differently to the same treatment. Apart from the cancer itself, every patient is different. Some may be relatively fit while others suffer from multiple chronic diseases. It is another reason why bespoke treatment is needed, based on precision diagnosis and an informed conversation between patient and physician.
Even cancers with the same site of origin, like lung cancer, can be very different from one person to the other – and respond differently to the same treatment.
But while the concept of personalized care has become a cornerstone of oncology research and cancer treatment over the last few decades, it remains riddled with challenges. Multidisciplinary care teams struggle with bringing together and keeping track of all relevant information pertaining to the patient – from a variety of subspecialty reports to information about the patient’s current health condition and history, as well as life circumstances. Deciding on the right therapy is equally complex. As our understanding of the heterogeneity of cancer has grown, so has the number of treatment options. Physicians and patients face a bewildering number of choices. Yet major progress is being made. In a series of two articles, of which this is the first, we will explore how recent advances in oncology research and new technological innovations are shaping the future of personalized cancer care. Central to these advances is the notion of integrating cancer care around patients throughout their journey. At every step in this journey – from precision diagnosis to treatment selection and execution – care teams need easy access to all relevant information in order to make informed decisions together with the patient. I believe this is how we can truly personalize cancer care, giving every patient the care that gives them the best chance of the desired outcome.
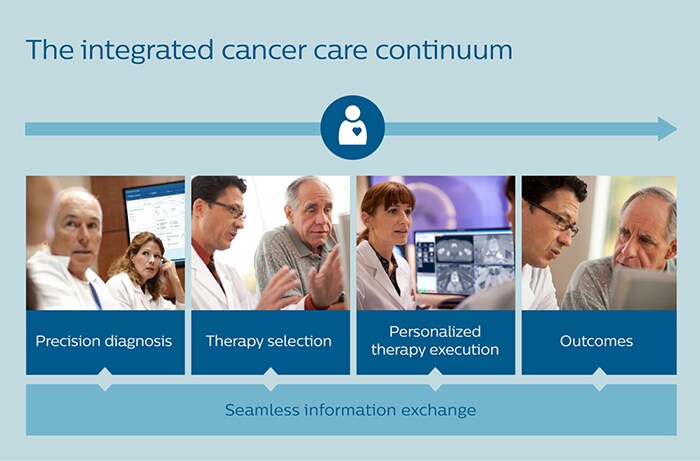
In this first article, lung cancer care will serve as a case in point.
It all starts with early detection
Lung cancer is the most common cancer worldwide, accounting for 2.1 million new cases in 2018 [1] and a cost impact of more than $80 billion in the U.S. alone [2]. While incidence rates are slowly declining due to smoking cessation (which is crucial to disease prevention), lung cancer will continue to pose a huge burden on healthcare systems in the foreseeable future [3]. The outlook for patients is generally grim, with less than 20% of patients surviving five years or more [4]. One of the main reasons for this low survival rate is that lung cancer is often detected late, when treatment options are limited. 80% of patients with lung cancer are already in an advanced stage when diagnosed, with cancer having spread out to lymph nodes in the chest or other areas of the body [5]. If lung cancer is found at an earlier stage, people have a better chance of survival. That is why early detection is critical – before further diagnostic procedures can take place to understand the unique characteristics of the patient’s cancer. Over the last decade, low-dose CT screening has gained prominence as a way of identifying possible early signs of lung cancer in the high-risk segment of the population. In 2011, the National Lung Screening Trial in the U.S. showed that this type of screening can decrease lung cancer mortality by 20% [6]. However, accuracy of low-dose CT screening leaves much to be desired. 96% of people who screen positive for lung cancer actually do not have a malignant lesion [7].
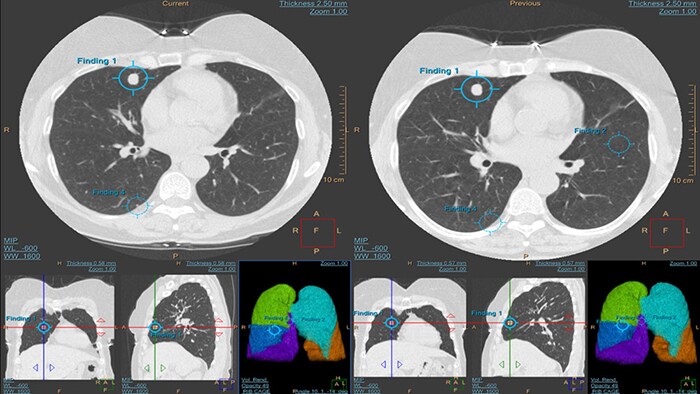
Low-dose CT screening can help with early detection of lung cancer, and machine learning can help to make that screening more accurate.
By training an algorithm on more than 6,000 cases, scientists at Philips have shown that machine learning may be helpful to radiologists in lung cancer screening as a decision support tool or second opinion [8]. Similarly, a study by the University of Pittsburgh showed that a machine learning algorithm can help to cut false positives by one third without missing a single case of cancer [9], preventing unnecessary anxiety and procedures in those patients. Further validation in a clinical setting is needed, but these early research results speak to the potential of AI to improve the predictive value of low-dose CT screening.
Attacking cancer cells with targeted therapies
Once malignant cell growth in the lungs has been established, a much deeper understanding of the tumor is required for precision diagnosis and personalized treatment. Historically, lung cancer was divided into a small-cell and non-small-cell type, a distinction that was used to guide treatment decisions. But today, we know that this dichotomy fails to capture the multifaceted nature of the disease. While two tumors may look similar in a microscopic image, they can be quite different on a molecular level. That is why analysis of the genetic makeup of a tumor is now an integral part of precision diagnosis. Genomic testing helps to identify the mutations in the DNA of cancer cells that drive the growth of the tumor. With this knowledge, it is possible to selectively attack proliferating cancer cells using targeted drug therapies, sparing healthy tissue. This stands in contrast to conventional chemotherapy, which attacks fast-growing cells whether they are cancerous or not, leading to damaging side effects. Although not all patients are eligible for targeted therapies yet, these therapies have significantly improved survival rates and quality of life in selected subgroups of patients with lung cancer [9].
While two tumors may look similar in a microscopic image, they can be quite different on a molecular level.
Decoding the molecular mechanisms of cancer
More recent research has revealed that we need to probe even deeper into the molecular mechanisms driving tumor growth. Mapping DNA mutations is useful, but does not give the full picture. It is like reading the concept for a book (the genetic code), when you also want to read the book itself (how the cancer actually forms and grows). If we look beyond the genome, DNA mutations are translated into RNA. This in turn triggers interactions among molecules such as proteins and enzymes. Determining the activity of these so-called signal transduction pathways is what holds the key to understanding cancer growth. We now know that all cancers, including lung cancer, are driven by approximately twelve different signal transduction pathways [10]. Each of these pathways can be targeted with specific drugs. The challenge, therefore, is to identify which pathways show aberrant activity in an individual. A new innovation from Philips Research called OncoSignal helps to do just that. By analyzing RNA expression in a tumor sample, it helps to unravel which activated pathways give rise to a person’s cancer. In the future, tests based on OncoSignal could help to select the optimal targeted drugs for personalized treatment [11].
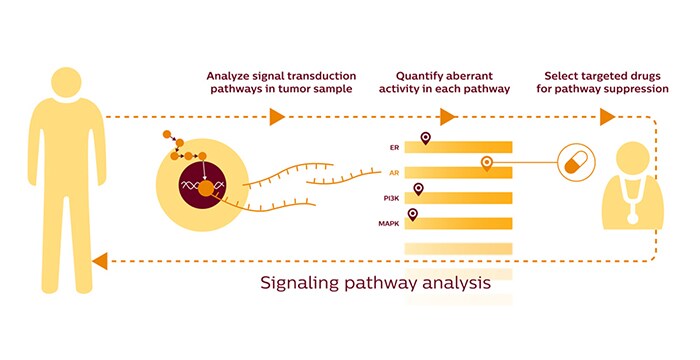
Bringing all the pieces of the puzzle together
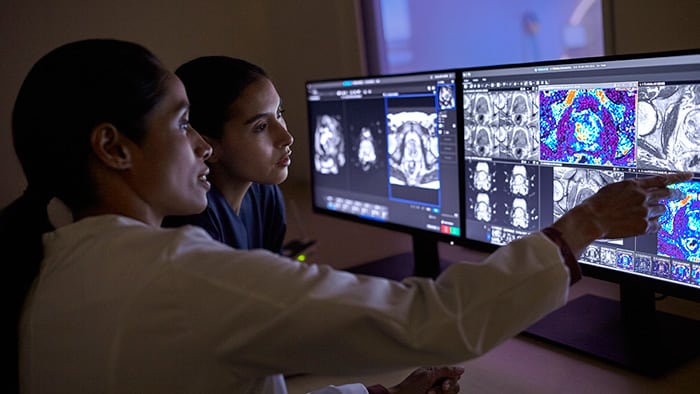
As we continue to unravel the inner workings of lung cancer, more and more pieces of the puzzle of a patient’s disease mechanism will be available to care teams. Medical images. Pathology reports. Genomics. Molecular test results. But all these pieces of data will be of little avail for precision diagnosis if it is hard to share them among specialists and bring them together into one patient overview. Everyone needs to be looking at the full puzzle, at every point in time. This is where multidisciplinary tumor boards often struggle today. Information that is generated in different parts of the hospital needs to be retrieved for joint decision-making; an often time-consuming process that is prone to error. Information may be incomplete or inconsistent. Consequences for the patient can be dire, for example if a malignant lung nodule is missed and doesn’t receive therapy. Speed is also of the essence. Every 14 days of delay in diagnosis lead to a 2 to 3% reduction in chances of survival [12]. As complexity of decision-making will grow only further, multidisciplinary tumor boards need quick and complete access to all relevant patient data. That is the philosophy behind Philips IntelliSpace Precision Medicine Oncology, a cloud-based platform designed to enable comprehensive patient-centric cancer care. It brings patient information together from disparate sources in dashboards that facilitate collaborative diagnostics, treatment and follow-up decisions by multidisciplinary tumor boards.

With Philips IntelliSpace Precision Medicine Oncology, different specialists have access to the same unified patient overview during a multidisciplinary tumor board meeting.
Personalized and evidence-based treatment suggestions
Even when multidisciplinary care teams have a common unified view of a patient for precision diagnosis, selecting the most appropriate treatment from an ever-expanding range of options can be a daunting challenge. How do you know which treatment will work best for a patient? To help answer this question, Philips last year announced a partnership with Dana-Farber Cancer Institute, a leading center of cancer research and treatment, to incorporate the institute’s Clinical Pathways in the IntelliSpace Oncology platform. With Dana-Farber’s Clinical Pathways, oncologists get recommendations for specific therapies – or clinical trials – matched to patient characteristics, such as specific gene mutations. Recommendations are based on the latest scientific insights and are continuously updated, enabling treatment selection that is more personalized and standardized at the same time. (Note that these clinical pathways for treatment selection are not to be confused with the signal transduction pathways that are at the origin of the cancer.)
Why patient context matters
Using proven Clinical Pathways does not imply that decision-making can be automated. The point is not to turn treatment selection into an “if this, then that” decision rule, but to support oncologists with relevant, evidence-based suggestions that can inform shared decision-making with the patient.
The point is not to turn treatment selection into an “if this, then that” decision rule, but to support oncologists with relevant, evidence-based suggestions that can inform shared decision-making with the patient.
Contextual factors, such as the fitness of the patient and possible comorbidities, are just as important to treatment selection (which is why we also make these visible in our IntelliSpace Oncology dashboards). For example, if a patient with lung cancer also has a heart condition, targeting the chest with radiation therapy to treat the lung cancer may be too risky. With a holistic understanding of the patient’s situation, the role of the oncologist will evolve into that of counselor. Together with the patient, the oncologist is best able to weigh the potential benefits, risks and side effects of different treatment options, and their impact on quality of life. This cannot and should not be turned into a mechanistic process. Because ultimately, personalizing cancer care is not just about understanding the cancer itself or the molecular mechanisms that give rise to it. It is also about recognizing that a patient is much more than their cancer, and that when it comes to matters of life or death, there is not always one right answer. Read more in the next article in this two-part series, which explores how a personalized approach to cancer care can extend to treatment delivery and monitoring.
References
[1] World Cancer Research Fund. https://www.wcrf.org/dietandcancer/cancer-trends/worldwide-cancer-data [2] American Cancer Society. https://www.cancer.org/cancer/cancer-basics/economic-impact-of-cancer.html [3] Jeon J, Holford TR, Levy DT, et al. Smoking and Lung Cancer Mortality in the United States From 2015 to 2065: A Comparative Modeling Approach. Ann Intern Med 2018, 169:684–693. https://annals.org/aim/article-abstract/2706939/smoking-lung-cancer-mortality-united-states-from-2015-2065-comparative [4] National Cancer Institute. https://seer.cancer.gov/statfacts/html/lungb.html [5] Birring SS, Peake MD. Symptoms and the early diagnosis of lung cancer. Thorax 2005; 60:268-269. https://thorax.bmj.com/content/60/4/268 [6] Reduced lung-cancer mortality with low-dose computed tomographic screening. National Lung Screening Trial Research Team. N Engl J Med. 2011 Aug 4; 365(5):395-409. https://www.ncbi.nlm.nih.gov/pubmed/21714641 [7] Trajanovski, S, Mavroeidis, D, Swisher, L, et al. Towards radiologist-level cancer risk assessment in CT lung screening using deep learning. 2018. https://arxiv.org/abs/1804.01901 [8] Raghu VK, Zhao W, Pu J, et al. Feasibility of lung cancer prediction from low-dose CT scan and smoking factors using causal models. Thorax 2019; 74:643-649. https://thorax.bmj.com/content/74/7/643 [9] Rolfo, C, Passiglia, F, Ostrowski, M, et al. Improvement in Lung Cancer Outcomes With Targeted Therapies: An Update for Family Physician. The Journal of the American Board of Family Medicine 2015; 28 (1); 124-133 https://www.jabfm.org/content/28/1/124 [10] Vogelstein, B, Papadopoulos, N., Velculescu, V. et al. Cancer Genome Landscapes. Science. 2013 March 29; 339(6127): 1546–1558. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3749880/pdf/nihms496129.pdf [11] OncoSignal is available for research use only and not yet for use in diagnostic procedures. [12] Kanarek NF, Hooker CM, Mathieu L, et al. Survival after community diagnosis of early-stage non-small cell lung cancer. Am J Med. 2014;127(5):443–449. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4601577/
Share on social media
Topics
Author

Henk van Houten
Former Chief Technology Officer at Royal Philips from 2016 to 2022