Apr 20, 2020
Philips' First Quarter Results 2020
Philips delivers Q1 sales of EUR 4.2 billion, with 2% comparable sales decrease; income from continuing operations amounted to EUR 42 million and Adjusted EBITA margin was 5.9%
First-quarter highlights Frans van Houten, CEO: “The start of 2020 was marked by the COVID-19 outbreak, and we have mobilized our resources since January to address this unprecedented challenge. At Philips, we are focused on our triple duty of care: meeting critical customer needs, safeguarding the health and safety of our employees, and ensuring business continuity. I am very proud of the commitment, hard work and resourcefulness of our employees to keep Philips fully functioning, and I would like to thank them for that. COVID-19 significantly affected our results in this quarter. There was increased demand for our professional healthcare products and solutions, with comparable sales and order intake growth for the Connected Care and Diagnosis & Treatment businesses. Comparable order intake grew 23%, most notably in diagnostic imaging, hospital ventilators, and patient monitors. We are investing more than EUR 100 million to steeply ramp up our production volumes, in close collaboration with our suppliers and partners. At the same time, there was a significant decline in demand for our Personal Health portfolio and we saw Image-Guided Therapy procedures trending down as the quarter progressed. This resulted in a 2% comparable sales decrease and an Adjusted EBITA margin of 5.9% for the Group. The impact of COVID-19 gradually increased in the course of the first quarter, initially affecting our businesses in China and Asia Pacific starting late January, and subsequently affecting our businesses in the rest of the world from March onwards. On that basis, we expect that all our geographies will be impacted throughout the second quarter. This is expected to result in a steep revenue decline for our Personal Health businesses and a sizable high-single-digit decline for our Diagnosis & Treatment businesses, partly offset by a significant increase in revenue of our Connected Care businesses. Assuming we can convert our existing order book for the Diagnosis & Treatment and Connected Care businesses as planned, elective procedures normalize, and consumer demand gradually improves, we aim to return to growth and improved profitability for the Group in the second half of the year. Consequently, for the full year 2020 we aim to achieve a modest comparable sales growth and Adjusted EBITA margin improvement. Given the current uncertainty and volatility, we will not provide more specific guidance for 2020 at this time.”
Business segment performance The Diagnosis & Treatment businesses recorded 2% comparable sales growth, led by mid-single-digit growth in Diagnostic Imaging, partly offset by a low-single-digit decline for Image-Guided Therapy due to the postponement of elective procedures. Comparable order intake was in line with Q1 2019, with double-digit growth for Diagnostic Imaging offset by a double-digit decline for Image- Guided Therapy. The Adjusted EBITA margin increased to 6.3%, as growth and productivity were partly offset by an unfavorable mix. Comparable sales in the Connected Care businesses increased 7%, with double-digit growth in Sleep & Respiratory Care. Comparable order intake showed a very strong double-digit increase, driven by strong demand for patient monitors and hospital ventilators. The Adjusted EBITA margin increased to 9.8%, mainly due to growth and productivity. The Personal Health businesses recorded a comparable sales decline of 13%, with all businesses declining due to significantly decreased consumer demand, resulting in an Adjusted EBITA margin of 7.1%. Philips’ ongoing focus on innovation and strategic partnerships resulted in the following key events in the quarter: Cost savings In the first quarter, procurement savings amounted to EUR 36 million. Overhead and other productivity programs delivered savings of EUR 59 million. Executive Committee update Rob Cascella, currently Chief Business Leader of the Precision Diagnosis businesses and member of the Executive Committee, jointly responsible for the Diagnosis & Treatment segment together with Bert van Meurs, will take on the role of Philips’ strategic business development per May 1, 2020. He will remain a member of the Executive Committee. Kees Wesdorp, currently General Manager of Diagnostic Imaging, will succeed Rob Cascella in his current roles and become a member of the Executive Committee reporting to Philips CEO Frans van Houten. Frans van Houten: “I would like to express my gratitude for Rob’s considerable contribution to Philips since he joined the company in 2015. Under his leadership, the Diagnosis & Treatment businesses have achieved a major step-up on the quality front and pivoted to outcomes-driven solutions. Most recently, Rob established and led our Precision Diagnosis businesses and was jointly responsible for the Diagnosis & Treatment segment. I am pleased that Rob will lead Philips’ strategic business development, and that Kees will be his successor. Kees will join the Executive Committee with a strong accomplishment record, having led the transformation of the Diagnostic Imaging business by increasing customer and employee engagement, renewing the product and solutions portfolio, and improving profitability. I am confident that he will further build out the Precision Diagnosis businesses through the transformation to solutions, continuing to drive robust growth and increased profitability. Kees and Rob will be working closely to ensure a seamless transition, with formal handover on May 1 of this year.” Capital allocation Philips has a strong balance sheet and robust liquidity position. In view of the possible continued impact of the COVID-19 pandemic in 2020, Philips has taken the following measures to further enhance its liquidity position: Share buyback program As of the end of the first quarter of 2020, Philips has completed 50.3% of its EUR 1.5 billion share buyback program for capital reduction purposes that was announced on January 29, 2019. On March 23, 2020, Philips announced that the second half of the program will be executed through individual forward transactions, to be entered into in the course of 2020, with the settlement dates extending into the second half of 2021. Further details can be found here. Euro Medium-Term Note In the first quarter, Philips successfully placed EUR 500 million fixed-rate Sustainability Innovation notes due 2025 and EUR 500 million fixed-rate notes due 2030. Dividend Philips maintains its proposed dividend of EUR 0.85 per common share against the net income of 2019. The distribution of this dividend will be in shares only, instead of the currently proposed distribution in cash or in shares at the option of the shareholder. To that effect, Philips withdraws the dividend proposal that was already submitted to the Annual General Meeting of Shareholders to be held on April 30, 2020. Philips plans to convene an Extraordinary General Meeting of Shareholders expected to take place in the second half of June 2020, the agenda of which will include the revised proposal to declare a distribution of EUR 0.85 per common share, in shares only. The increase in issued share capital is expected to be offset by the share buyback program mentioned above. In line with the measures described above, the Supervisory Board and the members of the Board of Management have agreed that the 2019 Annual Incentive for the Board of Management will be paid out in shares instead of cash. More information on the realization of the 2019 Annual Incentive can be found in the Remuneration Report, as included in the 2019 Annual Report (p 70-71). Regulatory update Philips Philips continues to fulfill its obligations under the Consent Decree [1] and remains in dialogue with the US FDA. In connection with the COVID-19 pandemic, Philips is working with the FDA’s Emergency Response and Product Evaluation teams to provide them with relevant information, such as Philips’ production ramp-up plans for critical products and solutions to combat COVID-19. Philips is actively seeking and has obtained authorizations through the FDA’s Emergency Use Authorization (EUA) process for the expanded use of several of its devices during the COVID-19 public health emergency, including for the Philips Respironics E30 ventilator, which received authorization on April 8, 2020. [1] Under the Consent Decree, Philips continues to export its range of AED devices and manufacture and distribute its HS1/OnSite/Home automated external defibrillator (AED) model in the US. The company may also continue to service the AEDs and defibrillator/monitors provided that certain conditions are met and provide consumables and the relevant accessories.
Report First Quarter Results 2020 - Report Presentation First Quarter Results 2020 - Results Presentation Conference call and audio webcast A conference call with Frans van Houten, CEO, and Abhijit Bhattacharya, CFO, to discuss the results will start at 10:00AM CET, April 20, 2020. A live audio webcast of the conference call will be available through the link below. Q1 2020 – First quarter 2020 results conference call audio webcast More information about Frans van Houten and Abhijit Bhattacharya Click here for Mr. van Houten's CV and images Click here for Mr. Bhattacharya's CV and images
Visit our interactive results hub for more on our financial and sustainability performance over the past quarter, including the latest version of our dynamic Lives Improved world map.
About Royal Philips
Royal Philips (NYSE: PHG, AEX: PHIA) is a leading health technology company focused on improving people's health and enabling better outcomes across the health continuum from healthy living and prevention, to diagnosis, treatment and home care. Philips leverages advanced technology and deep clinical and consumer insights to deliver integrated solutions. Headquartered in the Netherlands, the company is a leader in diagnostic imaging, image-guided therapy, patient monitoring and health informatics, as well as in consumer health and home care. Philips generated 2019 sales of EUR 19.5 billion and employs approximately 81,000 employees with sales and services in more than 100 countries. News about Philips can be found at www.philips.com/newscenter.
Forward-looking statements and other important information
Forward-looking statements These factors include but are not limited to: changes in industry or market circumstances; economic and political developments; market and supply chain disruptions due to the COVID-19 outbreak; Philips’ increasing focus on health technology; the realization of Philips’ growth ambitions and results in growth geographies; lack of control over certain joint ventures; integration of acquisitions; securing and maintaining Philips’ intellectual property rights and unauthorized use of third-party intellectual property rights; compliance with quality standards, product safety laws and good manufacturing practices; exposure to IT security breaches, IT disruptions, system changes or failures; supply chain management; ability to create new products and solutions; attracting and retaining personnel; financial impacts from Brexit; compliance with regulatory regimes, including data privacy requirements; governmental investigations and legal proceedings with regard to possible anticompetitive market practices and other matters; business conduct rules and regulations; treasury risks and other financial risks; tax risks; costs of defined-benefit pension plans and other post-retirement plans; reliability of internal controls, financial reporting and management process. As a result, Philips’ actual future results may differ materially from the plans, goals and expectations set forth in such forward-looking statements. For a discussion of factors that could cause future results to differ from such forward-looking statements, see also the Risk management chapter included in the Annual Report 2019. Third-party market share data Statements regarding market share, including those regarding Philips’ competitive position, contained in this document are based on outside sources such as research institutes, industry and dealer panels in combination with management estimates. Where information is not yet available to Philips, those statements may also be based on estimates and projections prepared by outside sources or management. Rankings are based on sales unless otherwise stated. Use of non-IFRS information In presenting and discussing the Philips Group’s financial position, operating results and cash flows, management uses certain non-IFRS financial measures. These non-IFRS financial measures should not be viewed in isolation as alternatives to the equivalent IFRS measure and should be used in conjunction with the most directly comparable IFRS measures. Non-IFRS financial measures do not have standardized meaning under IFRS and therefore may not be comparable to similar measures presented by other issuers. A reconciliation of these non-IFRS measures to the most directly comparable IFRS measures is contained in this document. Further information on non-IFRS measures can be found in the Annual Report 2019. Use of fair value information In presenting the Philips Group’s financial position, fair values are used for the measurement of various items in accordance with the applicable accounting standards. These fair values are based on market prices, where available, and are obtained from sources that are deemed to be reliable. Readers are cautioned that these values are subject to changes over time and are only valid at the balance sheet date. When quoted prices or observable market data are not readily available, fair values are estimated using appropriate valuation models and unobservable inputs. Such fair value estimates require management to make significant assumptions with respect to future developments, which are inherently uncertain and may therefore deviate from actual developments. Critical assumptions used are disclosed in the Annual Report 2019. In certain cases independent valuations are obtained to support management’s determination of fair values. Presentation All amounts are in millions of euros unless otherwise stated. Due to rounding, amounts may not add up precisely to totals provided. All reported data is unaudited. Financial reporting is in accordance with the accounting policies as stated in the Annual Report 2019. Certain prior-year amounts have been reclassified to conform to the current year presentation. Effective Q1 2020, Philips has simplified its order intake policy by aligning horizons for all modalities to 18 months to revenue, compared to previously used delivery horizons of 6 months for Ultrasound, 12 months for Connected Care and 15 months for Diagnosis & Treatment. At the same time, Philips has aligned order intake for software contracts to the same 18 months to revenue horizon, meaning that only the next 18 months conversion to revenue under the contract is recognized, compared to the full contract values recognized previously. This change eliminates major variances in order intake growth and better reflects expected revenue in the short term from order intake booked in the reporting period. Prior-year comparable order intake amounts have been restated accordingly. This realignment has not resulted in any material additional order intake recognition in Q1 2020. Market Abuse Regulation This press release contains inside information within the meaning of Article 7(1) of the EU Market Abuse Regulation.
This document and the related oral presentation, including responses to questions following the presentation, contain certain forward-looking statements with respect to the financial condition, results of operations and business of Philips and certain of the plans and objectives of Philips with respect to these items. Examples of forward-looking statements include: statements made about the strategy; estimates of sales growth; future Adjusted EBITA; future restructuring, acquisition-related and other costs; future developments in Philips’ organic business; and the completion of acquisitions and divestments. By their nature, these statements involve risk and uncertainty because they relate to future events and circumstances and there are many factors that could cause actual results and developments to differ materially from those expressed or implied by these statements.
Topics
Contacts

Ben Zwirs
Philips Global External Relations
You are about to visit a Philips global content page
Continue
Martijn van der Starre
Philips Global Press Office Tel: +31 6 2847 4617
You are about to visit a Philips global content page
ContinueBusiness Highlights Q1 2020
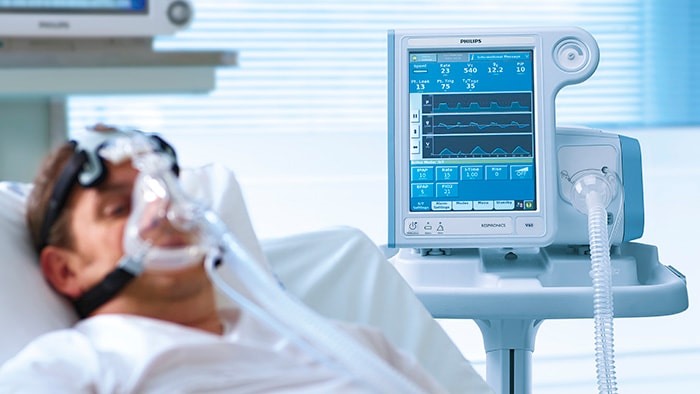
Philips details plans to increase its hospital ventilator production to 4,000 units/week by Q3 2020.

Jackson Memorial Hospital gives Philips Enterprise Monitoring as a Service model high marks for satisfaction and efficiency.

Philips starts producing new Philips Respironics E30 ventilator to help free up ICU units in wake of COVID-19.
Press releases
Get our press releases by e-mail
You are about to visit a Philips global content page
Continue







