Philips’ Xenon enabled MRI combined with XENOVIEW from Polarean can provide pulmonologists, surgeons and respiratory specialists with regional maps of ventilation in patients’ lungs to assist in better managing disease states. The first XENOVIEW clinical scan in North America recently took place at Cincinnati Children’s Hospital Medical Center on a Philips MRI system using the 510(k) cleared multi-nuclei imaging scanning module. Inhalation of an anoxic gas such as XENOVIEW may cause transient hypoxemia in susceptible patients. Patients should be monitored for oxygen desaturation and symptoms of hypoxemia and treated as clinically indicated. “This technology is groundbreaking and is already starting to make a difference to patients and providers, who want to know which parts of the lungs are functioning, which have obstruction, and how effective new therapies are,” said Dr. Jason Woods, Director of Pulmonary Research, Cincinnati Children’s Hospital and the University of Cincinnati. “The partnership between Philips and Polarean demonstrates a cutting-edge technique can be rapidly incorporated into a major MRI platform, without sacrificing quality.”
Philips has entered into a collaboration with medical imaging company Polarean to advance the field of hyperpolarized Xenon MRI for patients with respiratory illness. Philips will showcase its 3T MR 7700 system, featuring fully integrated multi-nuclei imaging, at the 2023 International Society for Magnetic Resonance in Medicine Annual Meeting & Exhibition (ISMRM 2023, June 3-8, 2023, Toronto, Canada). The Philips 3T MR 7700 and Polarean XENOVIEWTM (xenon Xe 129 hyperpolarized) enable an advanced solution for the evaluation of lung ventilation based on Xenon gas MR imaging, providing clinical confidence. XENOVIEW is indicated for use with magnetic resonance imaging (MRI) for evaluation of lung ventilation in adults and pediatric patients aged 12 years and older. Xenoview has not been evaluated for use with lung perfusion imaging [1].
Combining Philips MR 7700 multi-nuclei MRI scanner and Polarean’s XENOVIEW™ hyperpolarized Xenon contrast agent, the innovative solution may allow clinicians to view a patient’s lungs in greater detail, while also enabling them to accurately measure lung ventilation. For patients with pulmonary disease, it has the potential to make the difference between early diagnosis and intervention in serious obstructive lung diseases [1].
“With the MR 7700, we have seamlessly integrated multi-nuclei MR imaging into everyday workflows, making multi-nuclei studies of six different nuclei across all anatomies as simple as dragging and dropping the selected protocol onto an exam card. Our collaboration with Polarean to bring hyperpolarized Xenon imaging into the equation is a major breakthrough in improving the diagnosis and management of respiratory disease,” said Ruud Zwerink, General Manager of Magnetic Resonance and Digital X-Ray at Philips.
Our collaboration with Polarean to bring hyperpolarized Xenon imaging into the equation is a major breakthrough in improving the diagnosis and management of respiratory disease.
Ruud Zwerink
General Manager of Magnetic Resonance and Digital X-Ray at Philips
“Having attained FDA approval for the first and only hyperpolarized MR contrast agent with the launch of XENOVIEW at the beginning of 2023, we are excited to enter this agreement with Philips. Their strategic focus on patient and workflow centric multi nuclei imaging in their 3T system provides a uniquely capable platform for clinicians to extend their assessment of lung ventilation,” said Richard Hullihen, CEO of Polarean. “By deploying this novel technology to visualize otherwise unobtainable clinical information using MRI technology, MR imaging can now be expanded into pulmonary medicine, providing a quantitative tool to help clinicians and the patients they treat.”
In theory, any atomic element with a sufficient degree of magnetic polarization can be detected in an MRI scanner. However, unlike the hydrogen nuclei that conventional MRI scans image, the generated MR signal in most cases is extremely small. This is normally true for Xenon nuclei. So how is the combined solution used to acquire such detailed images of a patient’s lungs? The answer is two-fold: Polarean’s expertise in hyperpolarization physics and Philips’ expertise in MR image capture and reconstruction.
Leveraging unique capabilities in hyperpolarization physics, Polarean has perfected a practical means of transferring the energy in a circularly polarized laser beam to Xenon nuclei to create hyperpolarized Xenon gas, thereby increasing its MR signal by 10,000 fold [1]. XENOVIEW expands the opportunity for pulmonary medicine to utilize the first and only inhaled MRI hyperpolarized contrast agent for novel visualization of lung ventilation without exposing patients to any ionizing radiation and its associated risks [1]. This is particularly important in pediatric patients aged 12 years and older who may need to undergo multiple MR exams due to respiratory illnesses like cystic fibrosis.
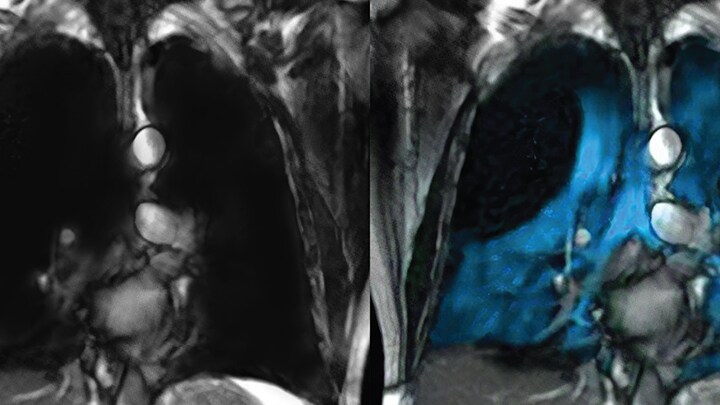
For patients with pulmonary conditions such as chronic obstructive pulmonary disease (COPD), asthma, cystic fibrosis, and bronchopulmonary dysplasia (BPD), evaluation of lung ventilation may allow detection of early/mild disease and assist in the monitoring of therapy. Compared to traditional CT scans, which can only image the first few branches of a patient’s airway, Xenon follows the same path as oxygen down to the small airways for visualization of gas distribution. [1] This content provided by the 3rd party, that remains solely liable for the fullness and correctness thereof. Any action or decision taken as a result of accessing this content or using websites linked to this content is at the discretion of the customer. Warnings and Precautions Risk of Decreased Image Quality from Supplemental Oxygen: Supplemental oxygen administered simultaneously with XENOVIEW inhalation can cause degradation of image quality. For patients on supplemental oxygen, withhold oxygen inhalation for two breaths prior to XENOVIEW inhalation, and resume oxygen inhalation immediately following the imaging breath hold. Adverse Reactions in Adult Patients: The adverse reactions (> one patient) in efficacy trials were oropharyngeal pain, headache, and dizziness.
For more information on Philips’ AI-driven smart connected imaging, optimized workflows, and integrated clinical solutions to improve MR productivity, enhance patient and staff experience, and deliver high quality diagnostic outcomes, including this latest collaboration with Polarean, visit Philips at ISMRM and SMRT 2023.
XENOVIEW IMPORTANT SAFETY INFORMATION
Risk of Transient Hypoxia: Inhalation of an anoxic gas such as XENOVIEW may cause transient hypoxemia in susceptible patients. Monitor all patients for oxygen desaturation and symptoms of hypoxemia and treat as clinically indicated.
Adverse Reactions
Adverse Reactions in Pediatric and Adolescent Patients: In published literature in pediatric patients aged 6 to 18, transient adverse reactions were reported: blood oxygen desaturation, heart rate elevation, numbness, tingling, dizziness, and euphoria. In at least one published study of pediatric patients aged 6 to 18 years, transient decrease in SpO2% and transient increase in heart rate was reported following hyperpolarized xenon Xe 129 administration. XENOVIEW is not approved for use in pediatric patients less than 12 years of age.
Please see full prescribing information at www.xenoview.net.
Share on social media
Topics
Contact

Kathy O'Reilly
Philips Global Press Office Tel.: +1 978-221-8919
You are about to visit a Philips global content page
Continue