Feb 20, 2019
Philips receives U.S. FDA 510(k) clearance to market DigitalDiagnost C90 digital radiography system with industry’s first live camera image at the tube head
Premium ceiling-mounted system supports accelerated patient throughput with innovative tools that help produce high quality images and drive workflow efficiency
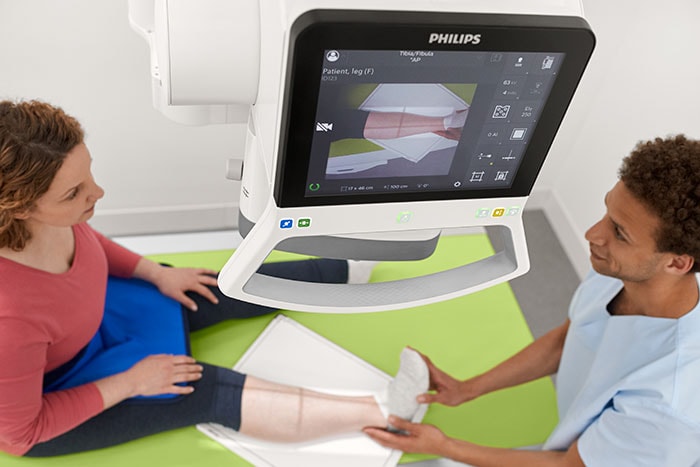
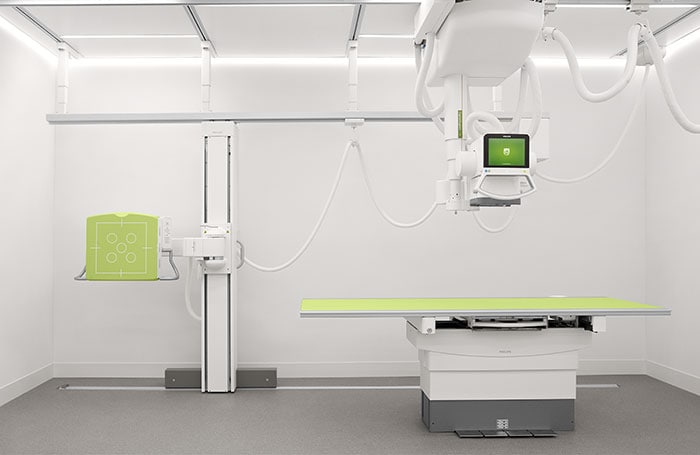
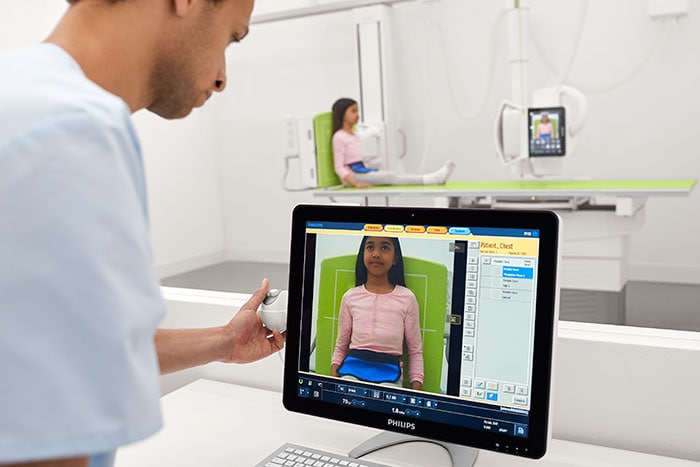
Amsterdam, the Netherlands – Royal Philips (NYSE: PHG, AEX: PHIA), a global leader in health technology, today announced it has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) to market the DigitalDiagnost C90, its newest premium digital radiography system. Designed to increase patient throughput and decrease the time to diagnosis, the Philips DigitalDiagnost C90 offers healthcare organizations a flexible and customizable imaging solution that helps to improve workflow and clinical outcomes, while adding economic value. X-ray is often the start of a patient’s care journey and plays a critical role in supporting clinical care decisions from that point forward – making high quality imaging essential. As the industry’s first radiography unit with a live camera image directly displayed at the tube head, DigitalDiagnost provides a clear view of the anatomical area being scanned during the patient positioning process – improving workflow so that clinicians can be confident that the right area is captured with a low X-ray dose exposure. With Philips’ UNIQUE 2 image processing and Bone Suppression software [1], radiologists can process clearer images for a more confident diagnosis with a lower chance of a costly and timely rescan. DigitalDiagnost C90 also incorporates the Philips Eleva user interface, a common platform across a range of Philips digital radiography systems that enables a smooth and efficient patient-focused workflow. This common user interface is now extended to the Eleva Tube Head, speeding up workflow by over 17% per examination [2]. Its touch screen display transfers operation into the examination room to allow for more time with the patient. DigitalDiagnost also helps contribute to a lower cost of care, with flexible room configuration options and SkyPlate sharing available among all Philips premium digital radiography systems.
With solutions like the DigitalDiagnost C90, Philips is helping radiology departments get one step closer to achieving that ambition through innovation that enables higher quality images, while keeping the patient and staff experience at the forefront.
Daan van Manen
Business Leader for Diagnostic X-ray at Philips.
“In today’s world of value-based care, healthcare organizations are investing in imaging solutions that help them achieve the quadruple aim: improved outcomes, enhanced patient experience, increased staff satisfaction and lowered cost of care delivery,” said Daan van Manen, Business Leader for Diagnostic X-ray at Philips. “With solutions like the DigitalDiagnost C90, Philips is helping radiology departments get one step closer to achieving that ambition through innovation that enables higher quality images, while keeping the patient and staff experience at the forefront.” The innovative design of the DigitalDiagnost C90’s Eleva Tube was recently recognized by the prestigious iF Design Awards in the categories of both product and service/user experience design. More information about Philips’ suite of digital radiography and fluoroscopy solutions is available here. [1] Riverain Technologies' ClearRead Bone Suppression. [2] Compared to a typical examination using the previous release of Philips’ DigitalDiagnost. Based on four images on average per examination. Validated by clinicians in a Philips development environment.
About Royal Philips
Royal Philips (NYSE: PHG, AEX: PHIA) is a leading health technology company focused on improving people's health and enabling better outcomes across the health continuum from healthy living and prevention, to diagnosis, treatment and home care. Philips leverages advanced technology and deep clinical and consumer insights to deliver integrated solutions. Headquartered in the Netherlands, the company is a leader in diagnostic imaging, image-guided therapy, patient monitoring and health informatics, as well as in consumer health and home care. Philips generated 2018 sales of EUR 18.1 billion and employs approximately 77,000 employees with sales and services in more than 100 countries. News about Philips can be found at www.philips.com/newscenter.
Topics
Contacts

Mark Groves
Philips Global Press Office Tel.: +31 631 639 916
You are about to visit a Philips global content page
ContinueMedia assets
Press releases
Get our press releases by e-mail
You are about to visit a Philips global content page
Continue