Aug 29, 2019
Philips debuts innovations in ultrasound and enterprise informatics to advance cardiac care at ESC 2019
Paris, France – Royal Philips (NYSE: PHG, AEX: PHIA), a global leader in health technology, is showcasing its latest cardiac care innovations at the ESC Congress 2019 (Paris, France, August 31 – September 4). At the congress, Philips is showcasing Release 5.0 of its EPIQ CVx cardiology platform for the first time in Europe. The platform includes automated applications for 2D assessment of the heart, as well as robust 3D right ventricle volume and ejection fraction measurements, making accurate exams faster and easier to conduct [1]. Philips also announced that it is collaborating with digital health company LindaCare to combine the company’s innovative OnePulse™ cloud-based solution for the remote monitoring of patients with cardiac implantable electronic devices (CIEDs) with the Philips IntelliSpace Cardiovascular informatics platform. “Monitoring and follow-up of cardiac arrhythmia patients with CIEDs can be often complex, with data trapped in different silos that need to be reviewed individually,” said Calum Cunningham, Business Leader for Enterprise Diagnostic Informatics at Philips. “LindaCare’s innovative OnePulse solution consolidates this data, and by incorporating OnePulse into our IntelliSpace Cardiovascular platform, caregivers can see this additional information as part of the broader cardiovascular history of each patient, allowing them to make the most appropriate treatment decisions for each patient.”
By incorporating OnePulse into our IntelliSpace Cardiovascular platform, caregivers can see this additional information as part of the broader cardiovascular history of each patient, allowing them to make the most appropriate treatment decisions for each patient.
Calum Cunningham
Business Leader for Enterprise Diagnostic Informatics at Philips
Philips IntelliSpace Cardiovascular is a web-based image and workflow management platform which streamlines the workflow of cardiology departments and across hospitals by consolidating multi-modality images and data and enabling access to an open ecosystem of cardiovascular software applications. The seamless combination of IntelliSpace Cardiovascular with LindaCare’s OnePulse solution allows clinicians to more easily access data from their patients’ CIEDs remotely. This results in a more seamless overall workflow, including alert management and triaging, supporting more proactive care. Philips is also introducing a new module on IntelliSpace Cardiovascular which complements the remote monitoring workflow by automating, standardizing and streamlining reporting for patients with CIEDs during hospital visits. Both the OnePulse interface and the new module will be available on the platform later this year. Since 2018, Philips has owned a minority interest in LindaCare through Philips Health Technology Ventures, which manages a business-agnostic digital health fund investing capital in future partners to help drive Philips’ digital transformation and jointly achieve the Quadruple Aim in healthcare.
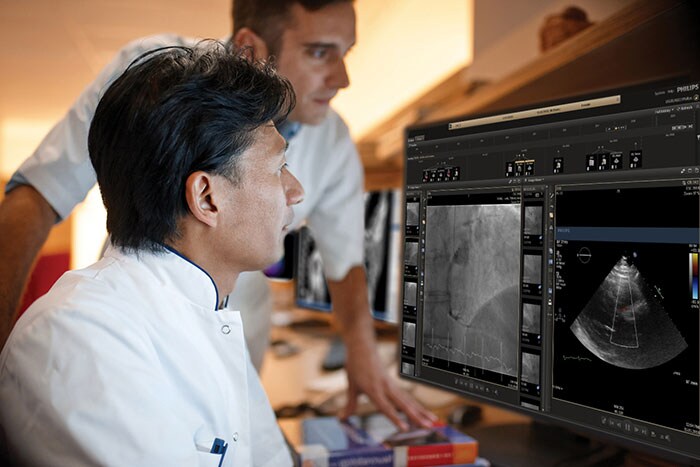
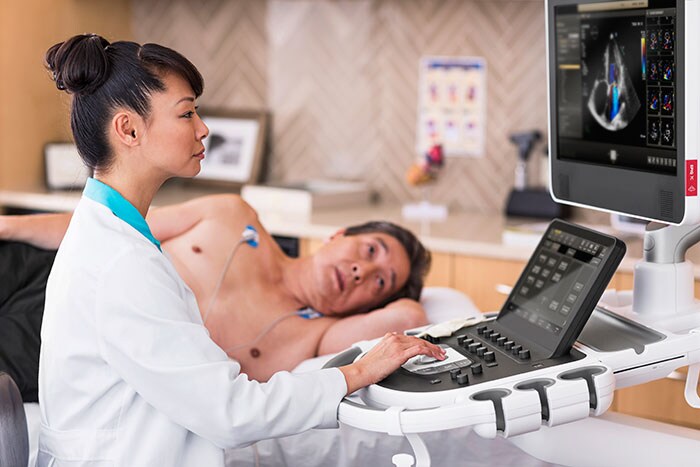
Increasing diagnostic confidence with the EPIQ CVx cardiology ultrasound platform
At the ESC Congress 2019 Philips is also highlighting the new advanced automation capabilities available on the EPIQ CVx cardiology ultrasound platform. By incorporating advanced automation, there is less variability between scans, leading to confident treatment decisions which benefits patients. The new release of EPIQ CVx is a major step forward, reducing the number of touches of the system by 21% in each exam, which is equivalent to more than 400 exams each year [2]. The AutoStrain LV application uses advanced Automatic View Recognition technology to identify the different views of the heart, providing exceptional visualization and analysis of left ventricular function – extremely important diagnostic information for patients at risk of developing cardiovascular disease. Also new are the AutoStrain LA and AutoStrain RV applications, which automate the measurement of left atrial and right ventricular longitudinal strain respectively. By creating reliable and reproducible strain measurements for the left ventricle, left atrium and right ventricle, the AutoStrain LV, LA and RV applications support clinicians treating patients with atrial fibrillation, arrhythmia and other complex heart conditions.
Enabling value-based cardiac care
With the shift to value-based care, healthcare providers are increasingly focused on balancing the need to provide the highest quality care with managing their operational costs. Philips provides intelligent solutions that help clinicians to be more efficient and effective, enabling them to deliver a consistent standard of care, optimize care pathways, simplify workflows and drive better treatment outcomes. The ESC Congress 2019 will take place at the Paris Expo Porte de Versailles, France. Visit Philips at Stand #590. During the event, Philips will host a Satellite Symposium on Ultrasound featuring leading worldwide experts. For more information about the symposium and other events, as well as general information about Philips’ presence at the show, visit www.philips.com/esc. [1] The Philips EPIQ CVx and EPIQ CVxi 5.0 Diagnostic ultrasound systems are available for sale globally. The AutoStrain LV, LA and RV applications are CE marked and FDA cleared and available for sale in Europe and in the USA. The AI enabled 3D AutoRV application, designed using a Machine Learning-based algorithm, is CE marked and FDA 510(k) pending, and is therefore available for sale in Europe but is not available for sale in the USA. [2] Based on eight scans per day over 48 weeks.
About Royal Philips
Royal Philips (NYSE: PHG, AEX: PHIA) is a leading health technology company focused on improving people's health and enabling better outcomes across the health continuum from healthy living and prevention, to diagnosis, treatment and home care. Philips leverages advanced technology and deep clinical and consumer insights to deliver integrated solutions. Headquartered in the Netherlands, the company is a leader in diagnostic imaging, image-guided therapy, patient monitoring and health informatics, as well as in consumer health and home care. Philips generated 2018 sales of EUR 18.1 billion and employs approximately 78,000 employees with sales and services in more than 100 countries. News about Philips can be found at www.philips.com/newscenter.