Dec 14, 2021
Philips to further expand its image-guided therapy devices portfolio through acquisition of Vesper Medical
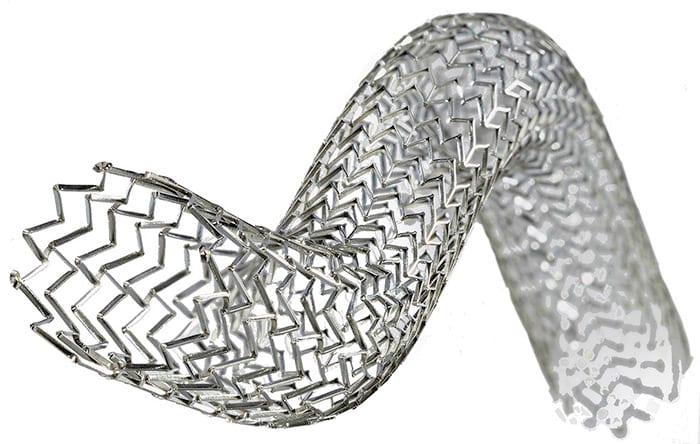
Amsterdam, the Netherlands – Royal Philips (NYSE: PHG; AEX: PHIA), a global leader in health technology, today announced that it has signed an agreement to acquire Vesper Medical Inc., a US-based medical technology company that develops minimally-invasive peripheral vascular devices. Vesper Medical will further expand Philips’ portfolio of diagnostic and therapeutic devices with an advanced venous stent portfolio for the treatment of deep venous disease. The transaction, which is subject to customary closing conditions, is expected to be completed in the first quarter of 2022. Financial details of the transaction were not disclosed. Philips already has a leading peripheral vascular portfolio, consisting of: live 2D/3D interventional imaging combined with intravascular ultrasound (IVUS) catheters to provide the visualization and guidance essential for the optimal diagnosis and treatment; peripheral atherectomy devices to remove blockages; and peripheral therapy devices, including the Stellarex drug-coated balloon to treat lesions, and the Tack implantable dissection repair device that restores blood flow in small limb vessels, which Philips added to its offering through the acquisition of Intact Vascular in 2020. With this portfolio, Philips provides clinicians with a complete procedural solution for the treatment of peripheral artery disease (PAD). Complementing Philips’ strong IVUS offering in venous imaging, Vesper Medical will add a venous stenting solution to address the root cause of chronic deep venous disease (DVD). The Vesper DUO Venous Stent System® consists of venous stents intended to treat deep venous obstruction. Uniquely engineered to address the multiple anatomical challenges of the deep venous system, it provides physicians with a modular portfolio to customize therapy, restore venous flow, and resolve the painful symptoms of deep venous disease for the broad range of patients suffering from chronic venous insufficiency.
The acquisition of Vesper Medical is another step in our objective to innovate patient treatment with more sophisticated technology and expand our growth in the vascular therapy space.
Chris Landon
Senior Vice President and General Manager Image Guided Therapy Devices at Philips
“The acquisition of Vesper Medical is another step in our objective to innovate patient treatment with more sophisticated technology and expand our growth in the vascular therapy space,” said Chris Landon, Senior Vice President and General Manager Image Guided Therapy Devices at Philips. “Leveraging our significant procedural expertise, we see strong clinical synergies between Vesper Medical’s innovative stenting solution and our existing peripheral vascular offering. This combined offering will help to better support clinicians to decide, guide, treat and confirm during the procedure, thereby enhancing patient care.” “I am proud that Philips will become the home for our innovations and our people, joining forces to shape the future of treating deep venous disease,” said Bruce Shook, President and CEO of Vesper Medical. “I look forward to further advance our next generation venous technology and bring it to patients and clinicians globally, together with Philips.” Vesper Medical was founded in 2016 and is headquartered in Wayne, Pennsylvania, US. Upon completion of the transaction, Vesper Medical and approximately 20 of its employees will become part of Philips’ Image-Guided Therapy business.
About Royal Philips
Royal Philips (NYSE: PHG, AEX: PHIA) is a leading health technology company focused on improving people's health and well-being, and enabling better outcomes across the health continuum – from healthy living and prevention, to diagnosis, treatment and home care. Philips leverages advanced technology and deep clinical and consumer insights to deliver integrated solutions. Headquartered in the Netherlands, the company is a leader in diagnostic imaging, image-guided therapy, patient monitoring and health informatics, as well as in consumer health and home care. Philips generated 2020 sales of EUR 17.3 billion and employs approximately 78,000 employees with sales and services in more than 100 countries. News about Philips can be found at www.philips.com/newscenter.
Forward-looking statements
This release contains certain forward-looking statements with respect to the financial condition, results of operations and business of Philips and certain of the plans and objectives of Philips with respect to these items. Examples of forward-looking statements include statements made about the strategy, estimates of sales growth, future EBITA, future developments in Philips’ organic business and the completion of acquisitions and divestments. By their nature, these statements involve risk and uncertainty because they relate to future events and circumstances and there are many factors that could cause actual results and developments to differ materially from those expressed or implied by these statements. CAUTION: Investigational device. Limited by Federal (United States) law to investigational use.
Topics
Contacts

Ben Zwirs
Philips Global Press Office Tel: +31 6 1521 3446
You are about to visit a Philips global content page
Continue
Derya Guzel
Philips Investor Relations Tel: +31 20 59 77055
You are about to visit a Philips global content page
ContinueMedia assets
Press releases
Get our press releases by e-mail
You are about to visit a Philips global content page
Continue