Philips launches real-time 3D intracardiac imaging in Europe, expanding access to minimally invasive heart procedures
May 19, 2025 | 3 minute read
Amsterdam, the Netherlands – Royal Philips (NYSE: PHG, AEX: PHIA), a global leader in health technology, today announced the introduction of its VeriSight Pro 3D Intracardiac Echocardiography (ICE) catheter in Europe. Building on its success in the United States, VeriSight Pro brings real-time 3D imaging directly inside the heart, helping physicians perform procedures with greater clarity — without the need for general anesthesia.
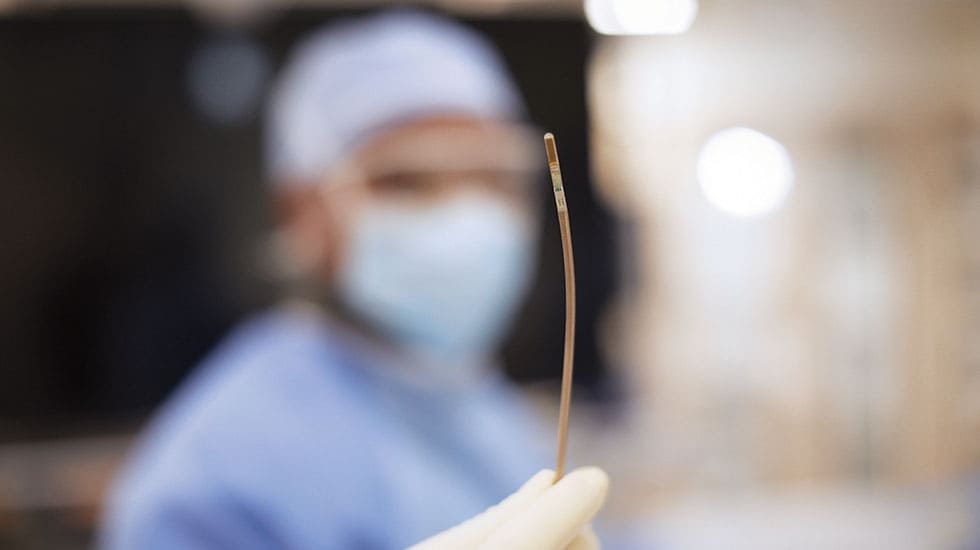
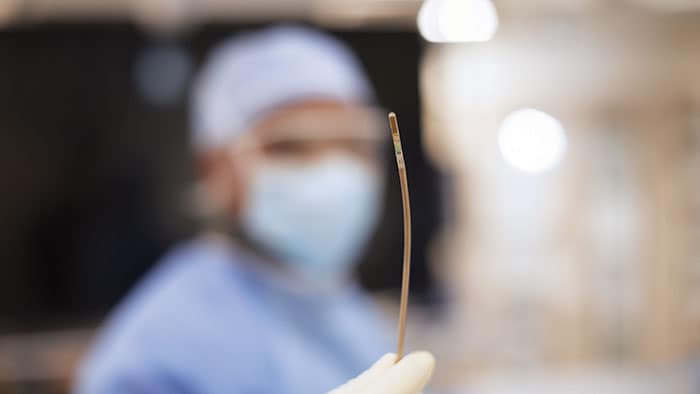
Designed for procedures such as transcatheter valve repair and left atrial appendage closure, VeriSight Pro offers high-resolution 2D and 3D visualization directly within the heart chambers. This enables confident clinical decision-making in structural heart and electrophysiology interventions, while removing the need for general anesthesia and associated risks.
With VeriSight Pro 3D ICE, we now have the ability to see detailed cardiac anatomy from inside the heart in real time.
“With VeriSight Pro 3D ICE, we now have the ability to see detailed cardiac anatomy from inside the heart in real time,” said Prof. Dr. Jörg Hausleiter, Ludwig-Maximilians-Universität (LMU) Munich, Germany. “This helps streamline our workflows and makes complex procedures more accessible to patients who may not tolerate more invasive imaging approaches.”
Addressing structural heart disease with less burden on patients and hospitals
Structural heart disease is a growing challenge across Europe, with increasing volumes of transcatheter valve repair and closure procedures. Today, many of these are supported by transesophageal echocardiography (TEE), which often requires general anesthesia, additional staff, and extended room time. In contrast, 3D ICE imaging provides detailed visualization from within the heart through a catheter introduced via the femoral vein, reducing the need for general anesthesia and recovery time in the Post-Anesthesia Care Unit (PACU), which can shorten hospital stays and lower costs.
VeriSight Pro is a pioneering innovation: a miniaturized ultrasound probe, approximately 3 millimeters in diameter, embedded at the tip of a thin, steerable catheter. This tiny device can be navigated through the vascular system and into the heart chambers, where it delivers high-quality 2D and 3D images in real time. Imaging the heart from within, with control over the scan angle, opens entirely new possibilities for guiding structural heart interventions. Physicians can assess anatomy, guide device placement, and confirm procedural results — all from a single access point, and without the need for more invasive imaging techniques. As the first ICE catheter to miniaturize the same 3D imaging technology used in TEE, VeriSight Pro helps address key barriers in delivering efficient, scalable care — from patient tolerance to resource availability in interventional suites.
“VeriSight Pro reflects our ongoing commitment to delivering clinically relevant innovations that enhance precision, reduce procedure complexity, and improve the care experience,” said Stacy Beske, Business Leader of Image-Guided Therapy Devices at Philips. “Its availability in Europe is an important milestone in helping more patients benefit from image-guided, minimally invasive heart procedures, while supporting care teams with integrated solutions that adapt to the way they work.”
Part of an integrated portfolio for structural heart care
VeriSight Pro is part of Philips’ structural heart disease ecosystem, seamlessly integrating with Philips’ EPIQ ultrasound systems, which in turn integrate with the Azurion image-guided therapy platform. Combined with tools such as EchoNavigator, Philips offers an end-to-end solution for clinicians performing procedures such as tricuspid and mitral valve repair and replacement, atrial septal defect closure, and left atrial appendage occlusion.
The catheter’s unique features — including xPlane and iRotate technologies — allow physicians to visualize two imaging planes simultaneously and digitally adjust views without physically repositioning the catheter tip. These capabilities enable precise assessment and device deployment with fewer imaging steps.
Showcasing 3D ICE in action at EuroPCR 2025
Philips will highlight VeriSight Pro and its role in structural heart interventions at EuroPCR 2025 in Paris, May 20–23. Key activities include: These sessions will offer clinicians an in-depth look at how 3D ICE is being applied in real-world structural heart procedures — from imaging protocols to workflow integration and clinical outcomes. A complete and detailed overview of sessions can be found here: www.philips.com/europcr.