It’s a disease that can leave people fatigued and short of breath even after simple day-to-day activities, and puts them at increased risk of stroke. Atrial fibrillation, or AFib for short, is a heart rhythm disorder that affects an estimated 33.5 million people worldwide – a number that is expected to more than double in the next 30 years as populations age and people live longer [1]. But despite impressive advances in medical technology, AFib is still difficult to treat effectively. Medication is usually prescribed, but may not be effective or tolerated by the patient. Fortunately, with minimally invasive image-guided procedures, it has become possible to restore normal heart rhythm – by ablating or neutralizing the heart cells that cause irregular contractions. Yet although these procedures have become increasingly sophisticated over the past two decades, they are not always successful the first time around. At least 20% to 40% of ablated AFib patients suffer from AFib recurrence [2]. New opportunities for treatment of AFib are now becoming available with the introduction of dielectric imaging – a breakthrough technology that allows electrophysiologists to see the heart like they have never seen it before. To understand what dielectric imaging is and how it can help electrophysiologists, let’s first have a look at how electrophysiologists perform AFib ablation procedures today and what challenges they run into.
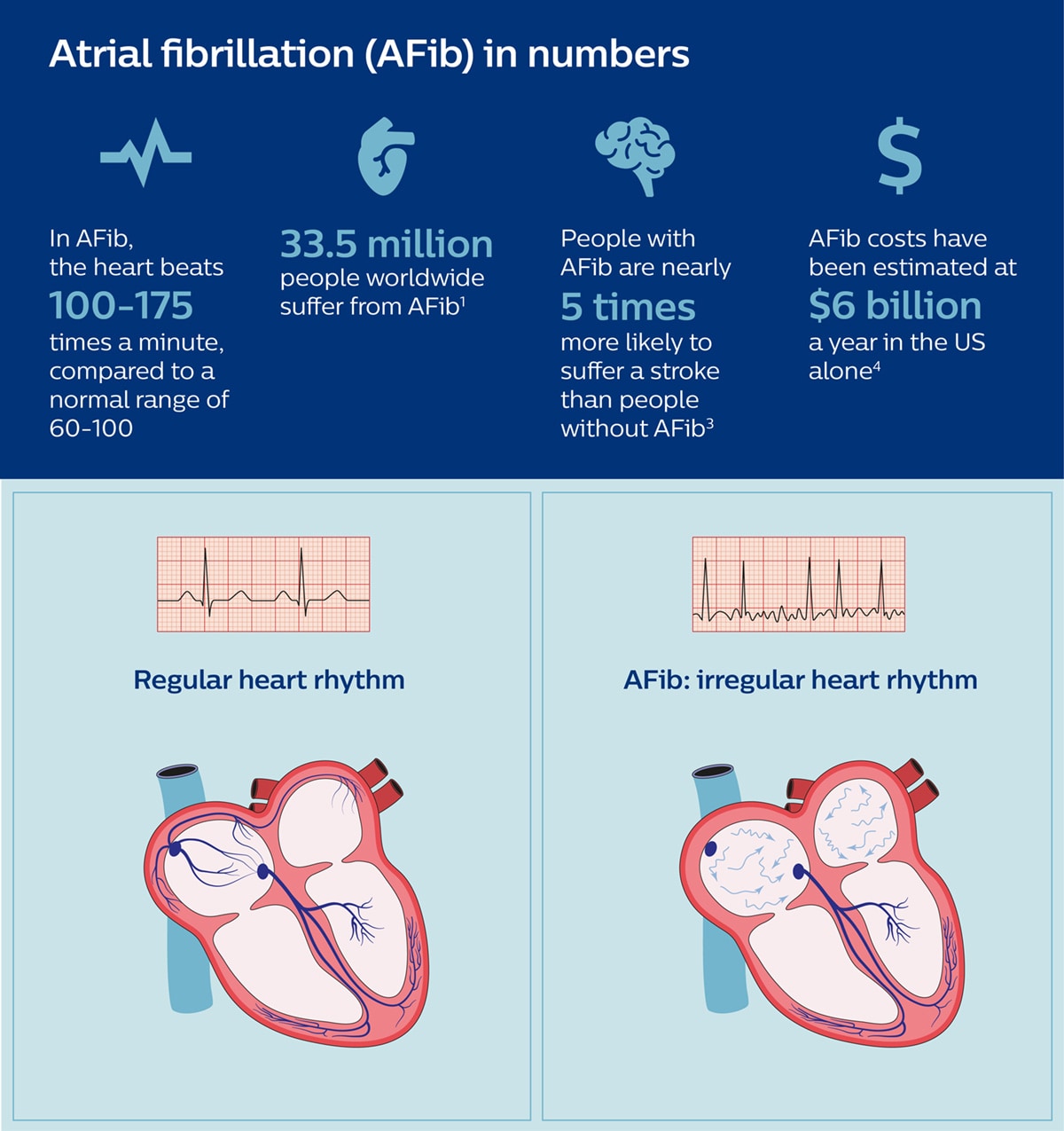
Resetting the rhythm of the heart
The mission of any electrophysiologist treating a patient with AFib is to get to the source of the electrical disturbances that cause irregular contractions of the heart – and to neutralize that source so that the heart will start beating normally again. Typically, AFib starts in the upper left chamber of the heart, with cells around the pulmonary veins that give off irregular electrical signals. By inserting ablation catheters into the groin and guiding them into the heart, small burns can be applied to neutralize or isolate those abnormally firing cells, using either extreme heat (radiofrequency ablation) or extreme cold (cryoablation) – thereby restoring normal electrical patterns and heart rhythm. A lot is at stake. The longer AFib goes unchecked, the worse it will usually get. Most worryingly: because AFib causes the heart muscle to quiver and pump out blood ineffectively, the disease can result in blood clot formation as blood pools inside the heart. When a blood clot travels to the brain, it can cause a potentially deadly stroke. Patients with AFib are nearly five times more likely to suffer a stroke than those without the condition, and AFib-related strokes are nearly twice as fatal as non-AFib-related strokes [3].
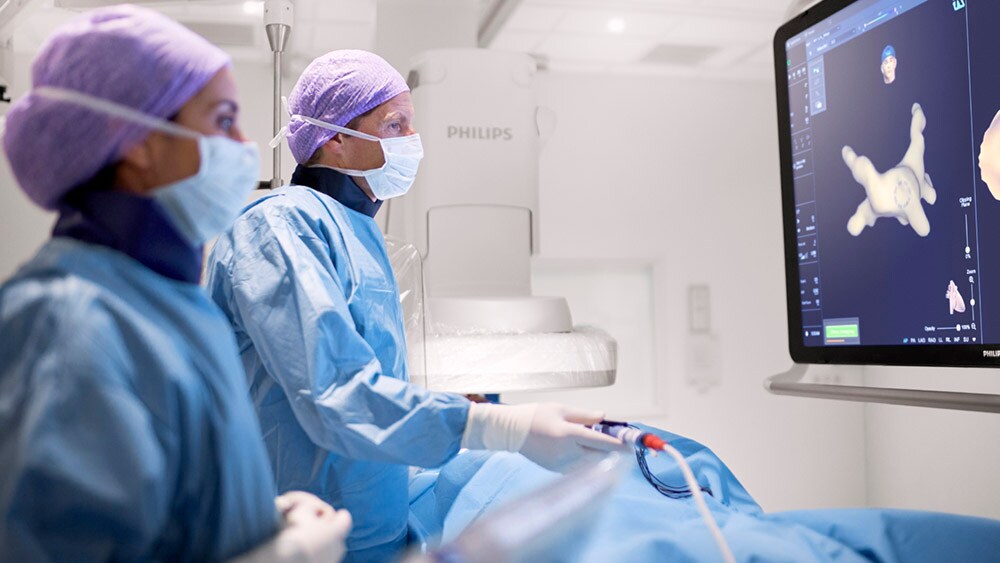
However, performing a minimally invasive ablation procedure is no easy feat. It requires the electrophysiologist to maneuver catheters through the heart with utmost precision, while it is beating (an extra degree of complexity that is hard to appreciate when looking at static images of the heart). Add to that the natural variability in cardiac anatomy from one patient to the other, and it becomes clear to see why an ablation procedure can take anywhere from 1.5 to 4 hours.
Performing an ablation procedure requires the electrophysiologist to maneuver catheters through the heart with utmost precision.
On top of that, there is no way to determine whether all abnormally firing cells were successfully neutralized. It only takes one tiny area of tissue to be left insufficiently treated for AFib to recur within a couple of months – like a small crack in a dam that slowly leaks until the dam bursts open. So how can dielectric imaging make a difference?
A new way of viewing the heart in high-definition 3D
Dielectric imaging, which we are introducing via our KODEX-EPD system, is based on a mechanism that is both surprisingly simple and radically different from how existing cardiac imaging and mapping methods work. Unlike traditional imaging methods such as CT and X-ray fluoroscopy, dielectric imaging doesn’t use radiation to visualize anatomical structures. And unlike traditional mapping systems, it doesn’t require catheters to touch the heart wall to visualize it. Instead, dielectric imaging exposes human tissue to weak electrical fields. Body sensors and electrophysiology catheters transmit electrical field waves into the patient’s body and heart. Because different types of cells have different physical properties, they will respond differently to the electrical waves. As the body sensors and the catheters inside the heart detect these varying signals, they are able to reconstruct what the anatomy around them looks like – imaging the heart from the inside out.

Using dielectric imaging, electrophysiologists can create detailed 3D views of the heart of a patient in as little as two minutes. This helps them navigate to the target location in the heart more easily and efficiently, with the precision that is needed, without using radiation.
Using dielectric imaging, electrophysiologists can create detailed 3D views of the heart of a patient in as little as two minutes.
Reducing radiation exposure for staff and patient
Reducing exposure to radiation and contrast agent is especially relevant to AFib patients, given the typical length of ablation procedures and the fact that some AFib patients have kidney dysfunction [5]. Because dielectric imaging offers high-definition 3D image quality during the procedure [6], it could also do away with the need for pre-procedural CTs and MRIs – saving patients an extra visit to the hospital. For the electrophysiologist and their staff, who may perform up to five procedures a day, a reduced need for X-ray fluoroscopy during the procedure contributes to a safer work environment – supporting our vision of reducing radiation exposure in the interventional suite as much as possible. But the possibilities of dielectric imaging don’t end there.
Towards real-time therapy assessment
Where dielectric imaging has the future potential to really go beyond existing mapping and localization systems is by offering insight into structural characteristics of heart tissue and the impact of ablation. The ultimate aspiration? Allowing electrophysiologists to assess in real time whether ablation in an AFib patient was fully successful or not. If electrophysiologists were able to spot possible signs of incomplete or ineffective ablation directly after performing the procedure, while the patient is still on the table, they may be able to take corrective action to reduce the risk of AFib recurrence. This could go a long in way in preventing the need for repeat procedures, limiting the burden on patient and staff while saving avoidable costs.
The ultimate aspiration is to allow electrophysiologists to assess in real time whether ablation was fully successful or not.
It shows our commitment to innovating an increasingly broad range of minimally invasive procedures, with integrated solutions that help deliver on the Quadruple Aim – enabling clinicians to perform procedures more efficiently and effectively, while offering an improved experience to both staff and patient. Add to that the future possibility of real-time therapy assessment, and we are moving one step closer to first-time right treatment. For people with AFib undergoing ablation, it could mean an increased chance of being able to quickly resume their normal life, with a normal heart rhythm, without having to return to hospital for a repeat procedure a few months later. A goal worth pursuing! References [1] Chugh SS, Havmoeller R, Narayanan K, et al. Worldwide epidemiology of atrial fibrillation: a Global Burden of Disease 2010 Study. Circulation. 2014;129(8):837–847. [2] Darby AE. Recurrent Atrial Fibrillation After Catheter Ablation: Considerations For Repeat Ablation And Strategies To Optimize Success. J Atr Fibrillation. 2016;9(1):1427. [3] https://www.stoptheclot.org/about-clots/afib-2/ [4] https://www.cdc.gov/dhdsp/data_statistics/fact_sheets/fs_atrial_fibrillation.htm [5] Hijazi, Z, Wallentin, L. Renal function in atrial fibrillation: a multifaceted dilemma. Circulation. 2016;134(1):48-51. [6] Maurer T, Mathew S, Schluter M, et al. High-Resolution Imaging of LA Anatomy Using a Novel Wide-Band Dielectric Mapping System. JACC: Clinical Electrophysiology. 2019 Aug 28.
Share on social media
Topics
Author

Bert van Meurs
Chief Business Leader of Image Guided Therapy, Chief Business Leader of Precision Diagnosis (ad interim) Bert van Meurs is Chief Business Leader of Image Guided Therapy, Chief Business Leader of Precision Diagnosis (ad interim), and is responsible for Diagnosis & Treatment. He is also a member of the Royal Philips Executive Committee.
Follow me on













