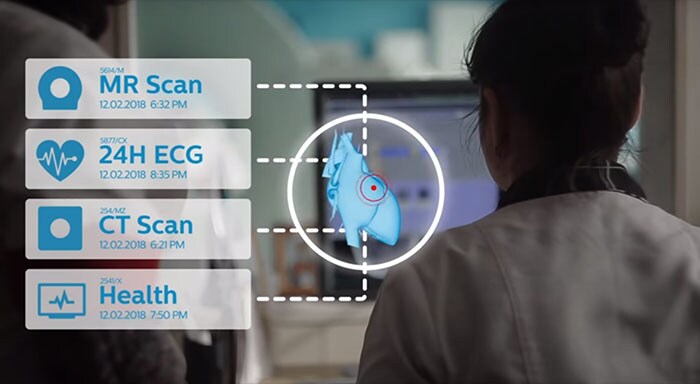
In the parable of the blind men and the elephant, six blind men encounter an elephant in their village. Each of them touches a different part of the animal, but only one part, such as the side or the tusk. When the men describe their findings to each other, they seem inconsistent. The men argue vehemently – until a wise man tells them they are all accurate, but incomplete. In medicine, a field that is becoming more and more specialized, this age-old parable serves as a reminder of the importance of getting the whole picture. Different data may point to different conclusions when viewed in isolation. Medical practitioners should ideally have an integrated understanding of a person’s health. [1] Could the ‘digital patient’ – a digital twin of the human body – be the means to this end? Digital twins are proving to be a powerful paradigm for personalizing healthcare and making it more effective and efficient, as I outlined in my two previous posts. Digital twins of medical devices can help to predict maintenance needs and test new products by using simulations. Similarly, patient-specific digital models of human organs, such as the heart, can support with diagnosis, treatment planning and guidance. The notion of a digital patient takes this one step further: instead of having isolated models of different organs, a digital patient or ‘health avatar’ integrates every relevant piece of medical knowledge about you. [1,2] A digital patient is a lifelong, integrated, personalized model of a patient that is updated with each measurement, scan or exam, and that includes behavioral and genetic data as well. How can we turn this vision into reality, and what considerations should we keep in mind? That’s the central question in this third post. But first, let’s explore how patients could benefit from having a digital double – and how these digital twins could support healthcare providers.
Moving towards personalized and preventative healthcare
Like an electronic health record, a digital patient collates individual health information from different sources in one place. But a digital patient is more than just a static digital record. It integrates and analyzes every bit of information – like a smart assistant that accompanies patients and their caregivers along the patient journey. As it is updated over time, it provides intelligent advice to support medical decisions and to help patients manage their disease.
A digital patient is more than just a static digital record. It integrates and analyzes every bit of information over time – like a smart assistant that accompanies patients and their caregivers along the patient journey.
As an example, take heart failure: a chronic disease that is the leading cause of hospitalization in patients over 65 years. [3] There is no cure, but with the right medication, patients can often be kept in a stable condition in the comfort of their homes for a longer time. However, there is a 50% chance of hospital readmission within six months, [4] diminishing patients’ quality of life and leading to mounting costs as populations age. The challenge with diagnosing heart failure is that the disease does not come with a few strong indicators. Rather, it has many weak and unspecific symptoms in different parts of the body – ranging from abnormal heart sounds to shortness of breath and swollen ankles (see figure below).
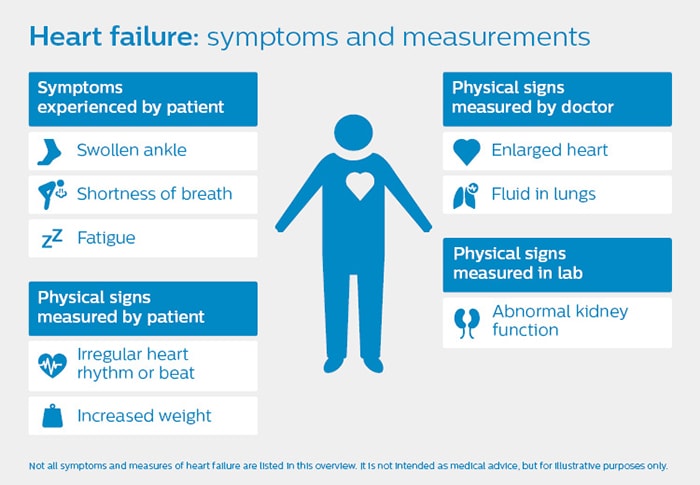
Just as it is hard to recognize an elephant by only feeling its side or tusk, you cannot assess heart failure based on one symptom alone. It is the combination of symptoms that matters. Could we build an intelligent monitoring system to detect early signs of deterioration in heart failure patients and reduce the number of re-hospitalizations? At Philips Research, we are developing computational models that could support such a system. A general practitioner (GP) would be able to get a better understanding of the patient’s current condition, based on physiological measurements that are automatically integrated. The system could also provide the GP with recommendations – for example, to adjust the patient’s medication, to perform additional measurements on the patient, or to consult a cardiologist. Though not a clinical reality yet, this example shows the promise of the digital patient paradigm. It has the potential to make healthcare more precise, personalized, and preventative, by equipping caregivers with the right insights at the right time.
The digital patient paradigm has the potential to make healthcare more precise, personalized, and preventative, by equipping caregivers with the right insights at the right time.
Patients would also have access to their digital twin, for example via a simple interface on their smartphone, to get more insight into their condition, to log health data or symptoms, and to get bespoke lifestyle tips. At a population level, aggregating anonymized data from thousands of digital patients could contribute to increased understanding of diseases – allowing for better targeted treatment and prevention programs, as well as better informed public health decisions. The strength of the digital twin paradigm is that it combines scientifically proven knowledge with biophysical modelling and insights derived from data. It builds on all the wisdom that the medical profession has accumulated, and adds the power of AI and data analytics to it. Of course, the feasibility of this vision hinges on the availability of high-quality data. And this is where the road gets rocky.
The biggest hurdles: unstructured, incomplete data, and lack of data sharing
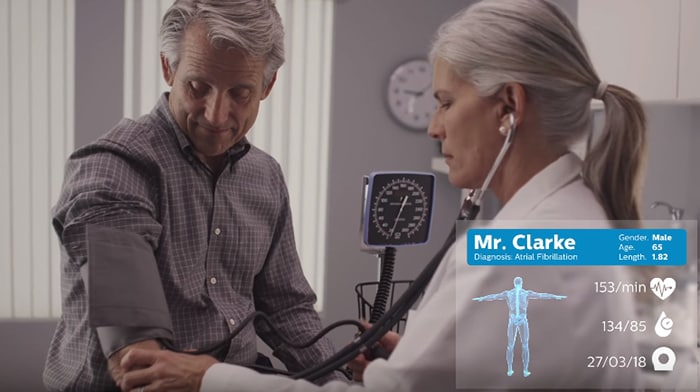
One fundamental difference between a digital twin of a device and a digital twin of a person is that the former starts logging data immediately and does so continuously, whereas the latter has to be compiled and matured over time. Unlike a machine, a person is not born with a blueprint of their inner workings. A person’s digital twin slowly takes shape ̶ starting from a generic, scientifically underpinned model, and fleshed out more fully with health data that is collected intermittently over a person’s life. Adding to the complexity is that as much as 75% of healthcare data is captured in an unstructured way, in medical notes of various kinds, residing in different systems. As one GP observed, “the biggest hurdle is to obtain relevant data and a common understanding of what the data represents.” [5] To bring the vision of the digital patient to life, we need shared standards and platforms for collecting and exchanging healthcare data. For example, in monitoring patients with heart failure, a subjective measurement in a GP’s office – such as listening to the heart via a stethoscope – could eventually be replaced by a more objective measurement, such as a basic assessment of heart functioning via a portable ultrasound scanner. A cardiologist in a nearby hospital would need easy access to the same data, so patient care can be orchestrated via a shared digital model of the patient. As this example shows, taking clinically relevant measurements regularly – rather than intermittently – is also a crucial part of the digital patient paradigm. Data acquisition will increasingly move from hospitals to the GP and the home environment. A cardiologist could delegate certain routine measurements to a GP. And, at the discretion of the GP, a patient may be able to perform certain measurements at home, such as taking blood pressure. The digital patient paradigm requires us to rethink the collection of health data as a continuous, collaborative process with the patient at the center.
Data acquisition will increasingly move from hospitals to the general practitioner and the home environment.
Will we ever have a full digital patient?
As medical science progresses, it is not hard to see how we will be able to integrate ever more data into our digital twins – bringing together information on an anatomical, biomolecular, behavioral, and genetic level, to generate insights that help improve people’s health and quality of life. But how far can we and should we take the concept of the digital patient? Some have raised doubts over whether a digital patient with sufficient fidelity is within the grasp of medical science, pointing to the complexity of the human body. [5] Indeed, we should recognize that the human body is infinitely more complex than the most elaborate machine. It consists of sub-systems that interact in manifold ways, and the number of variables that influences your health is potentially endless. Unlike engineered objects, which are more or less identical by design, people are very different from each other too. This puts constraints on what we realistically can expect to predict even with the most advanced models. Critics have also pointed out that in our endeavors to tailor prevention and treatment ever more closely to the individual patient, the improved outcomes may not always weigh up to the costs. In some cases, they argue, there may be more obvious and cheaper interventions that don’t require much individual data – such as a generic lifestyle program focused on diet and exercise. [6] Furthermore, there is a concern that continuous monitoring of health data could lead to over-diagnosis, or even over-treatment. These reservations serve as a reminder that the digital patient is a tool, and not a goal in itself. The goal remains to improve the quality of patient’s lives. We need to develop digital models around concrete and manageable clinical challenges – always weighing the investment against the expected benefits, and focusing on the most relevant data. Medical specialists should be strongly involved in these endeavors, because they have the domain knowledge that is so indispensable in creating valid and useful models. Physicians know how to make sense of noisy and incomplete data; something that will always be hard for computers and AI. The resulting digital models should fit seamlessly into their workflows, with intelligence that adapts to their needs and preferences.
Medical specialists should be strongly involved in any digital patient initiative. They have the domain knowledge that is so indispensable in creating valid and useful models.
Putting the patient in control
As we continue on this path, one crucial question remains: who owns the digital patient? Clearly, the digital patient paradigm calls for strong data governance, including measures that ensure security and transparency of data usage.[7] This requires careful design – particularly as the digital patient will aggregate information longitudinally, over many episodes of care, involving multiple healthcare providers. Ultimately, there is only one person who should be able to decide how your health data is used. And that person is you. Because at the end of the day, that is what the digital patient paradigm is all about: improving lives by putting people at the center of healthcare. Physically, and virtually.
References
[1] Roadmap for the digital patient (2016). http://www.vph-institute.org/upload/discipulus-digital-patient-research-roadmap_5270f44c03856.pdf [2] Brown, S. (2015). Principles for developing patient avatars in precision and systems medicine. Frontiers in Genetics, 6, 365. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4705226/ [3] Díez-Villanueva, P., & Alfonso, F. (2016). Heart failure in the elderly. Journal of Geriatric Cardiolology, 13(2), 115–117. [4] High heart failure readmission rates (2017). http://heartfailure.onlinejacc.org/content/5/5/393 [5] Raden, N. (2018). Digital twins for personalized medicine: promising, with caveats https://siliconangle.com/blog/2018/04/20/digital-twins-personalized-medicine-promising-caveats/ [6] Interlandi, J. (2016). The paradox of precision medicine. https://www.scientificamerican.com/article/the-paradox-of-precision-medicine/ [7] Bruynseels, K., Santoni De Sio, F., & van den Hoven, J. (2018). Digital twins in health care: ethical implications of an emerging engineering paradigm. Frontiers in Genetics, 9, [31]. http://pure.tudelft.nl/ws/files/38853007/fgene_09_00031.pdf
Share on social media
Topics
Author

Henk van Houten
Former Chief Technology Officer at Royal Philips from 2016 to 2022